Burning Desire for Efficiency
Posted by JoulesBurn on June 28, 2012 - 6:32pm
This is a guest post by Tom Murphy. Tom is an associate professor of physics at the University of California, San Diego. This post originally appeared on Tom's blog Do the Math.
 Ever wonder how efficient it is to heat water? Of course you have! Ever measured it? Whoa, mister, now you’ve gone too far!
Ever wonder how efficient it is to heat water? Of course you have! Ever measured it? Whoa, mister, now you’ve gone too far!
I recently devised a laser-phototransistor gauge to monitor my natural gas meter dial—like ya do. As a side benefit, I acquired good data on how much energy goes into various domestic uses of natural gas. Using this, I was able to figure out how much energy it takes to heat water on the stove, cook something in the oven, or heat water for a shower. Together with the knowledge of the heat capacity of water, I can compute heating efficiency from my measurements. What could be more fun? I’ll share the results here, some of which surprised me.
Heating Basics
The amount of energy it takes to heat water is so well-established, that it is the basis for several prominent units of energy. For instance, the calorie is the amount of energy it takes to heat one gram (1 mℓ) of water by 1°C. As a straightforward extension, 1 kcal = 4184 J (often Calorie with capital C) is how much energy it takes to heat one kilogram (or liter) of water by 1°C. Likewise, 1 Btu = 1055 J is the amount of energy it takes to heat one pound of water by 1°F.
So if I want to take 500 mℓ of water from 18°C to boiling, I need to expend 82×0.5 kcal to get the job done, or 171.6 kJ.
Measuring the Gas
My natural gas meter usually receives little attention from me—which is saying something for a person as measurement/data crazed as myself. The reason is that the meter does not provide sufficient information to track small expenditures without vigilant monitoring. As explained in the pilot lights post, there are dials that make revolutions once every half-cubic-foot and two-cubic-feet, then a jump to 1000 cubic feet. The jump is so large that one cannot walk up to the meter and know at a glance how many wraps the high-resolution dials have made since the last look.
Rather than parking myself outside for days on end to keep track of my gas gauge, I bought a cat-toy laser pointer and modified it to be powered by a constant 5-volt power supply. I aimed the laser at the ½-cf dial so that the black needle would interrupt the beam once per revolution. Then I set up a photo-transistor to “watch” the laser spot, and tuned the sensitivity so that the needle would make a robust change in the photocurrent. The laser and detector were shoved into a crudely drilled block of plywood to point at the same spot on the dial face, and then clamped to the meter. A dishtowel provided ambient light baffling, and a plastic shopping bag gave it some modest protection from rain and a certain trashy look. Thankfully, our gas meter information is now digitally transmitted. I can’t think what the meter reader’s reaction would be to walk up on this clap-trap arrangement (glowing red at night, no less).

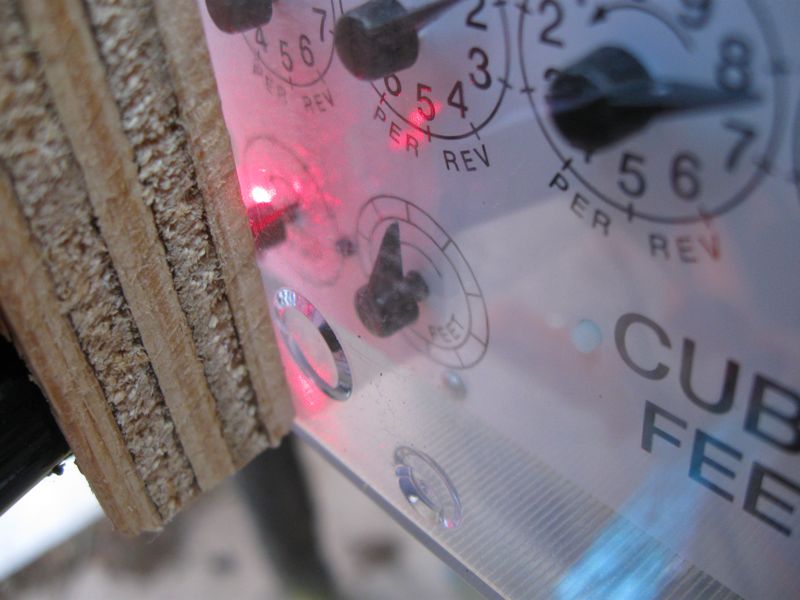
I kept the electronics in the garage (only the laser and sensor were outside), accompanied by a data acquisition unit and a computer to log the continuously sampled series (3 second samples were sufficient). Not the most elegant way to get the data, but I used stuff I had on hand. And it worked. And the plots are nice.
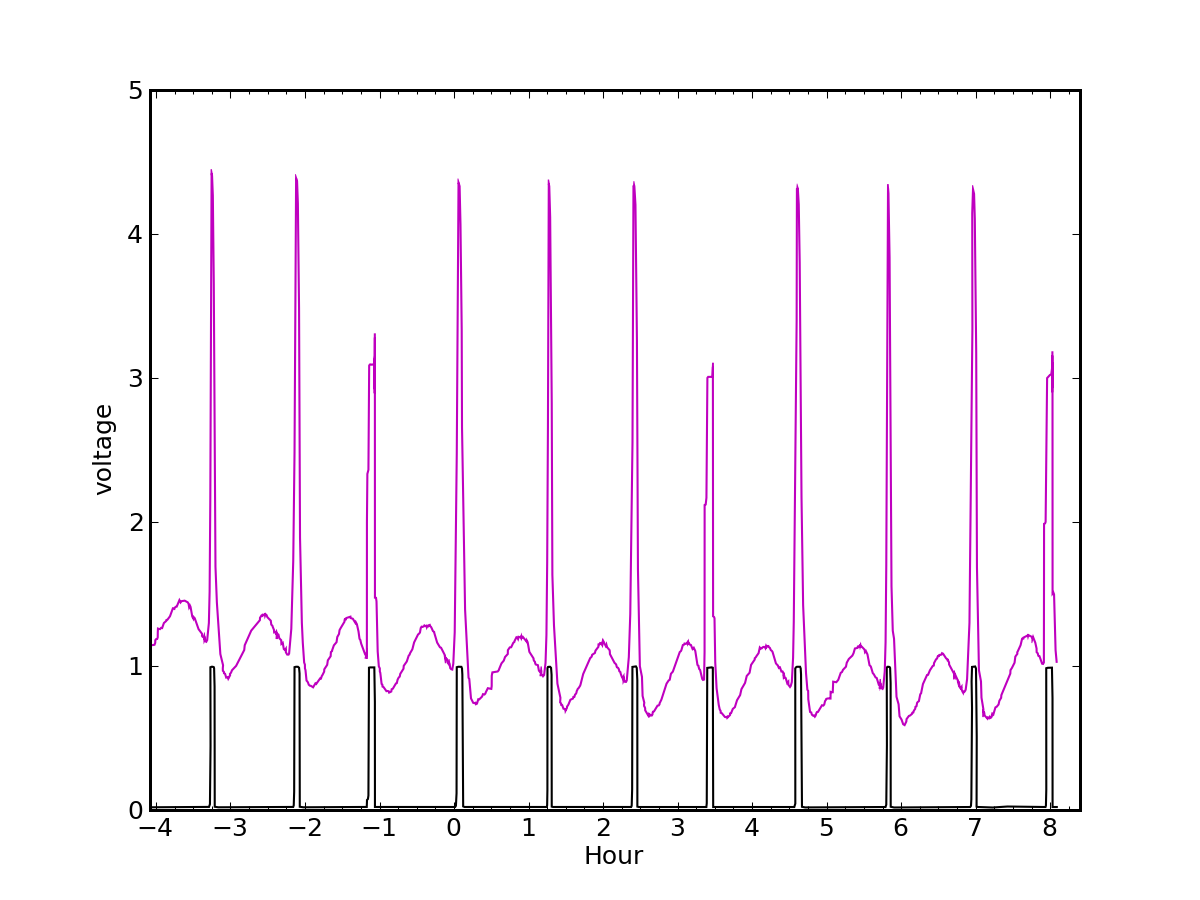
Most of the time, the water heater pilot light creates our only gas demand. In the summertime, even our shower activity—albeit modest—is satisfied by the pilot. We can see in the plot above the periodic signature of the needle interrupting the laser, and also a sinusoidal behavior between needle crossings. Unintentionally, the sinusoid gives a useful cue as to exactly when a high-flow demand (stove, oven, heater) is initiated, so that one does not have to wait until the needle event for the first indication. The sinusoidal pattern is presumably from scattered light off of irregularities in the needle hub.
Even without the extra benefit from the hub, one can determine how much gas was used by noting how many cycles appear between the languid pilot spikes, compared to how many revolutions the pilot would have produced in the same period.
Each half-cubic-foot of gas is equivalent to 510 Btu, since 100 cubic feet delivers 1.02 Therms, and a Therm is 100,000 Btu. Therefore, each turn amounts to 538 kJ, or 0.149 kWh.
Stove-top Boil
Enough with the preliminaries. I lured you in with promises of efficiency measurements, and went off instead into some mad scientist tangent.
If I place 500 mℓ of water in a copper-bottomed 4-quart (approx. 4 ℓ) pot, and set this pot—without lid—on top of the largest burner and let-er rip, what efficiency do I get? The flames are mostly hitting the bottom of the pot, but maybe a smidgeon of curling around the sides. Any guesses?
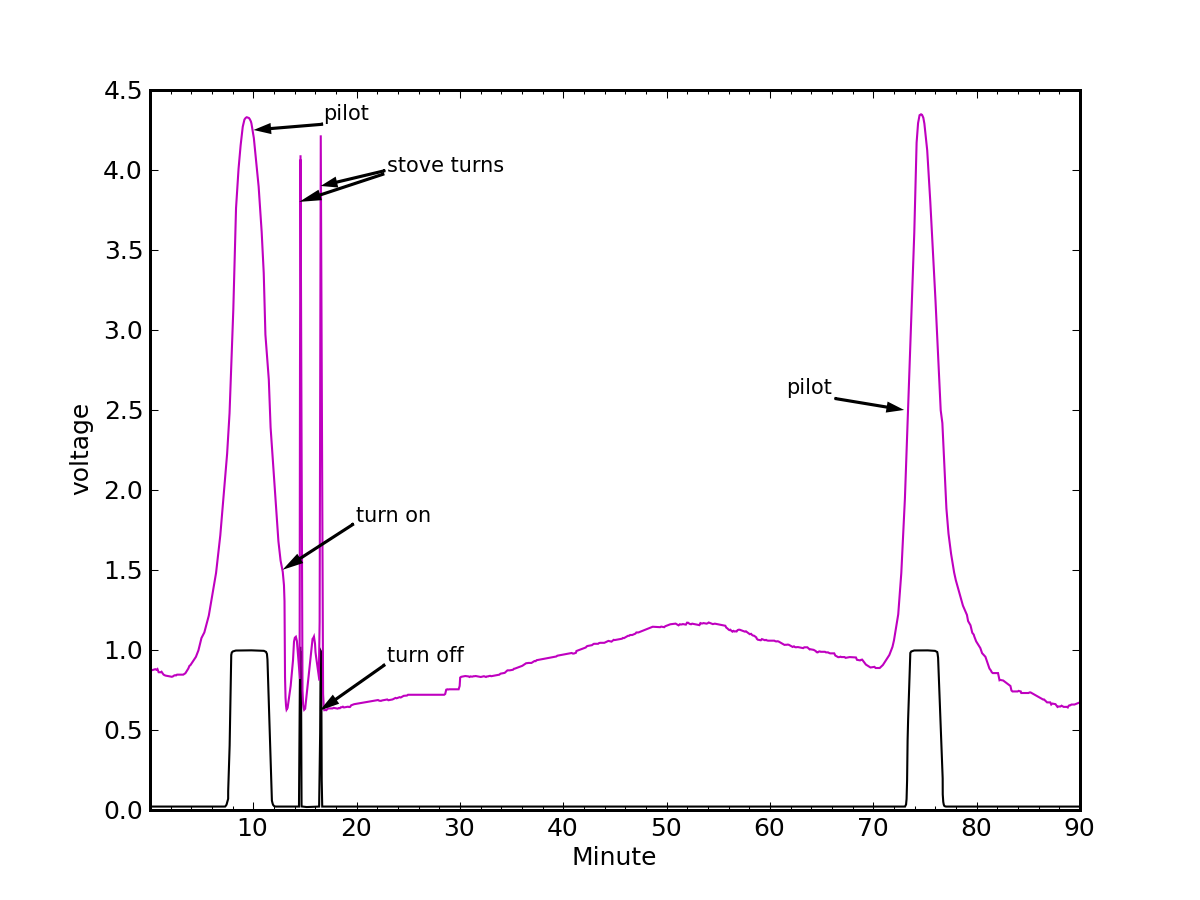
Starting from an ambient temperature of 18°C (water at equilibrium), and heating to a boil, the gas meter reveals 1.09 MJ of energy was used on top of the steady burn from the hot water heater pilot light, at a rate of 4500 W (about 15,000 Btu/hr). Meanwhile, it was supposed to take only 171.5 kJ of energy to boil this amount of water. So the efficiency was a mere 16%.
I was surprised. Are you surprised? I mean, burning the fuel is itself highly efficient. The pot sits right atop the flame. Dirt simple. Some heat escapes around the side, some goes to heating the pot itself, but come-on—16%?!
In another test on another night, I boiled 500 mℓ of water starting at 16.5°C on a smaller burner (1670 W, or 5,700 Btu/hr). I used the same pot, but this time with its lid. I put the burner on full power, but its smaller diameter meant that no flames visibly licked around the bottom of the pot. Want to guess as to the efficiency in this mode? I deem this mode to be about as efficient as I am likely to get on the stove-top using standard practices. The answer: I used 640 kJ of gas for something that should have taken 175 kJ for an efficiency of 27%. An enclosed kettle on the same burner delivered the same result.
Okay, so a 68% improvement over the full-throttle approach is a significant gain (although it took almost twice as long). But still, I was surprised that I top out at less than 30% on a gas stove burner.
Incidentally, from the rate of cooling after the burner was turned off, I gather that the water remaining in the open pot lost heat at a rate of about 300 W through convection, radiation, and possibly some conduction to the grate. These mechanisms are roughly proportional to ΔT between the water and the environment, so on average come to about 150 W during the linear ramp-up of temperature from ambient to boiling. This would have cost 63 kJ in the slow case (about 10% of the total), or about 35 kJ in the full-blast case (3% of the total). But the lidded case would actually lose heat less quickly than these numbers indicate, as the loss rate is based on an open pot after the boiling test was complete. In either case, loss from the pot to the environment appears to play a relatively minor role.
How About Microwaves?
Okay, the stove top delivered disappointing efficiency results. But right above the stove, I have a microwave. Naively, I expected the microwave to be something like 80% efficient at delivering energy to the water. The thinking goes like this: the interior of the microwave oven is a good reflector for microwaves, so they rattle around until they find an absorber; namely, the food/water. Of course there will be some loss in generation at the magnetron tube (I imagine it gets hot). And the walls may not be perfect reflectors, so that after twenty bounces, there may not be much energy left. But still, 80%—right?
This time, I put 500 mℓ of 18°C water into a plastic measuring cup (63 g) and heated on full power for 90 seconds, measuring the temperature afterwards. The water reached 50°C, demanding 67 kJ of energy. Meanwhile, both a Kill-A-Watt and TED (The Energy Detective) told me that I used 157 kJ of utility energy. That’s 43%, folks. Yes, I’m disappointed too. A later test at 120 seconds produced 60°C water (this time from 22°C) for a computed 40% efficiency.
Considering that electricity often comes from fossil fuels at 30–40% efficiency, the microwave (as tested by me) is only 15% efficient at transforming fossil fuel heat into heated water. That’s worse than the stove top—especially the slow-and-steady version.
Electric Kettles
What about electric kettles, where a heating element is immersed into the water, directly heating the thing you care about? To boot, these kettles often have insulated sides, keeping the heat where you want it.
I borrowed a kettle from work, and heated 500 mℓ of water from 18°C. I was able to suspend a thermometer in the water in a way that did not compromise the lid or spout. After 152 s, the water was making plenty of happy bubbling noise and the thermometer read 100°C—although apparently it was not as well-calibrated as I would have expected, since it sailed up to 105°C after 173 s. The water was obviously in a vigorous boil by then, and I shut the kettle off at 210 s (I should have let it turn off on its own, but lost patience with its inefficient lethargy).
Both the Kill-A-Watt and TED agreed that the kettle consumed about 1440 W. So while I needed 0.5×4184×82 = 171.5 kJ to raise the water to boiling from 18°C, I spent 219 kJ by the time the (erroneous) thermometer read 100°C; 249 kJ by the time a full, roiling boil was obvious; and 302 kJ by the time I shut the device off. Using the middle number, I calculate 69% efficiency. I get 78% if I use the shortest time, when I thought I was at boil. But by the time I shut it off, we were down to 57%, and falling. Who knows how far it would have sunk before shutting itself off (kicking myself that I don’t know).
If the shutoff is sufficiently sensitive, it seems electric kettles are capable of something like 70% efficiency or better. It would seem to beat the pants off the other methods—except for two caveats.
The first is the one raised for the microwave. If your electricity comes from fossil fuels, 70% efficiency becomes 25% in turning fossil fuel energy into hot water. The stove top is competitive.
The second caveat is that kettles suffer a sometimes rather large inefficiency due to lax filling practices. A kettle is considered to be a reservoir. Few measure the amount of water they put in. As long as it’s enough, game on. So the tendency is to overfill. All the water gets heated. Only some is used. This practice is probably even more pervasive in communally-shared kettles, and could easily cut a deep hole into the net efficiency. By contrast, microwave practices typically heat exactly as much as is consumed. A kettle with 50% over-fill turning off on its own sweet time can easily sink to the levels achieved by the microwave.
Hot Water Heater
While we’re talking about the efficiency of heating water, how well does my gas-fired hot water heater perform in transferring combustion energy into the water tank? Seems like it should be pretty good: few places for the heat to go except into the water (although the flue does get super-hot).
Practically speaking, efficiency suffers from slow heat loss during idle times, and from loss through the pipe walls during delivery. Tankless applications can avoid these two loss mechanisms. But let’s separate these out and ask only about the direct efficiency with which combustion energy gets into the water in the first place. To do this, I poured hot water into a bathtub until the water heater came on. I let the heater complete its business, then waited about an hour, after which I drew another batch of hot water until the heater activated again. This way, the second heating did not have to compensate for long-term loss of heat from the reservoir.
Mind you, this experiment represented unusually excessive hot water use in our domicile, but I did it for the people. And we did manage to enjoy baths in the bargain.
I measured the temperature of the water emerging from the (hot only) tap, and from a tap right at the inlet from the street (adjacent to water heater, too). I used the water meter by the street to gauge volumetric use to a precision of 0.01 cubic feet (0.3 ℓ). And, of course, the gas meter told me how much energy was consumed.
On the first wave, I used 1.39 ft³, or 39 ℓ, heated from 22°C to 53°C (remind me to turn the heat down; I should also note that the water came out at 50°C on the first draw, but it had cooled in the tank for some unknown time). Multiplying these fine numbers by 4184 J/ℓ/K, I compute an energy demand of 5.1 MJ. Meanwhile, my gas gauge made 17.15 revolutions at 510 Btu/rev for a total of 9.2 MJ. The efficiency computes to 55%.
On the second withdrawal—this time based on a recently heated tank, it took 51 ℓ before the heater engaged, after which the gas dial made 19.15 turns. This time, the demand was 6.6 MJ, while the gas cranked out 10.3 MJ. The efficiency is 64%.
Again, I find myself disappointed. I need to re-adjust my intuition about how straightforward it is to channel heat from a flame into water on the other side of a metal wall.
Bonus Round: Gas Oven
I seriously doubt I’ll get around to another post detailing the things I learned from my laser-gauge gas meter. So I’ll stick in here what I learned about my gas oven. How much energy does it take to “charge” up? How much power to keep steady? How much “on” time equivalent does it take for the pre-heat phase?
I heated my oven to 425°F (218°C) from an ambient 20°C temperature. It took 9.5 minutes to arrive at the setpoint, during which time the half-cf dial on the gas meter made five turns. Correcting for the water heater pilot light rate, this action took 2480 Btu, or 2.6 MJ (0.72 kWh). The burner operated at about 4800 W (16,300 Btu/hr). Thereafter, I found that it took a steady 1500 W to maintain temperature: I left the oven unopened and undisturbed for the better part of an hour to make sure I reached equilibrium—all for the sake of experiment. This means that the preheat phase uses the same amount of energy as 30 minutes of steady operation. You don’t need fancy gauges to tell you this if you note the preheat time is 10 minutes and observe that the burner is on one third of the time during steady operation.
If you’re heating a pizza in the oven that requires ten minutes of cook time, then only 25% of the total energy is spent in cooking mode, the other 75% in preheat. If cooking something for an hour, the preheat surcharge drops to 15%.
Although I am embarrassed to reveal the efficiency of cooking pizza in the oven (since this is not unknown in my household), I owe it to myself to carry out the calculation. Let’s say I heat a 383 g pizza by 200°C (but that it’s heat capacity is in between that of water (at the high end) and more typical materials—say 2000 J/kg/K. So I need to inject 0.383·2000·200 = 153 kJ. Meanwhile, my oven heats up for ten minutes and then the pizza spends ten minutes in the oven. I count 2.6 MJ for preheat, and an additional 0.9 MJ for the cook time. In the end, I manage to get 4.4% of the expended energy into the pizza. If I instead tried 6 minutes of full-power equivalent in the microwave oven (at 1750 W), I might get near 25% efficiency—and a flaccid crust.
Summary Table
Lots of numbers thrown around in this post. Here’s a table of my results.
The adjusted efficiency is the fossil fuel equivalent if electricity is derived from fossil resources (coal, natural gas) at 35% efficiency. The range on the electric kettle depends on how quickly the kettle shuts off in response to boiling water.
Testing Without Lasers
If you wanted to replicate or extend these experiments, is it hopeless without the laser-sensor gauge I created? Not at all—although slightly less convenient. In fact, I carried out the hot water heater test after I had already dismantled the gauge. Here’s one trick. Once you’ve characterized the burn rate of various devices (e.g., stove burners on max flame; oven while burner is on; water heater; furnace; etc.) then all you need is a way to measure time when the (audible) burner is on.
As alluded to above, keeping track of the fraction of time the oven burner is on in steady operation is enough to tell you how much energy goes into preheat vs. holding temperature. Even without calibrating a stove burner, different configurations (lid, different pots, etc.) can be compared against each other just by timing.
To measure the rate of gas usage of various devices, make sure that device is the only thing on, and time how long it takes the half-cubic-foot (or 2 cf) dial to make one revolution. Together with knowledge that each half-cf translates to 510 Btu (538 kJ), you’re pretty much set. Having a way to measure volumes and temperatures also came in handy for me.
What of It?
Heating water is less efficient than I originally thought. All the same, it will always take 1 kcal to heat 1 kg of water 1°C, and heating water is something we will always be interested in doing. Yet, efficiencies are middling-enough that we can’t expect gigantic improvements. Heat pumps could break the 100% efficiency barrier (by a factor of several), but these are impractical for small-scale applications.
Should you react to the numbers above by rushing out to buy an electric kettle, with the promise of tripling the efficiency over the stove-top solution? In part, this depends on your source of electricity. If your electricity is derived from fossil fuels, and you otherwise have a gas stove, then it’s probably not worth it. But even if going from an electric stove to an electric kettle, we must consider the embodied energy of the kettle. I discussed two methods for estimating embodied energy in another post, which for this case results in something like 50 kWh of investment. Each cup (250 mℓ) of water takes 0.03 kWh of energy at 70% efficiency. If you’re tripling efficiency, then you save 0.06 kWh per cup, and need to boil over 800 cups before the thing pays for itself, energetically. Maybe this makes sense. But it’s not a burning imperative.
As with many things, a far more effective strategy is to first recognize how and why you use sources of energy. After developing an awareness, you are far less likely to heat excess water in a kettle that you don’t plan to consume. Shorter, less frequent showers can have a far bigger effect on your energy use than how you heat water for tea. It comes back to behaviors.
In the meantime, it’s kind-of nice to have some numbers for water heating efficiencies—as disappointing as the numbers themselves are.



If I miss a turn and have to drive my 15mpg klunker truck around the block, traveling, say, .15 miles out of the way, how many cups of tea do I have to forgo to make up the energy difference (assuming I heat my tea on the stove top, small burner with a lid and only heat up exactly one cup each time)? My guess is that missing a turn is way more costly than any way I could choose to be more efficient in boiling water. Am I right?
Probably depends on whether you miss that turn every day. If that is the case, you should probably refrain from boiling water.
LOL. We physicists like to measure stuff; and environmentalists and some energy experts like to sweat small stuff (like light bulbs.) The latter is partly on the excuse that small stuff adds up over big populations, but on another hand, big stuff would add up much faster. But on yet another hand, big stuff is often political dynamite, so it may be more congenial to sweat small stuff. (If you moralize at people about light bulbs, they may get ticked off, but if you moralize at them to waste two or three hours a day waiting for buses, they may get really ticked off.)
Now, as to the teacups. To heat, say, 200ml of tea from 20C to 100C will take roughly 80*4*200, or 64000 joules. It won't be perfectly efficient, and it will probably boil briefly (and wastefully), so call it 100000 joules. If it's electric heat, make that 200000 or 250000 joules of fuel; after all fuel is still the main ultimate electricity source in most places. On your numbers, the truck will consume 0.15/15, or 0.01 gallons, at, call it 135MJ/gallon, giving 1350000 joules of fuel. So maybe we're talking six cups of tea here.
Of course if it's a high-mileage car instead of a truck, one might get it down to 1½ or 2 cups of tea. So you'll have to decide for yourself if it's your cup of tea.
Great response! Thanks! I love the math/science aspects of energy, yet as an activist, I know that the solutions need to be regulations and laws on the production/infrastructure side of the equation. Consumer decisions are a red herring that allow corporations to put the onus of blame on others while they make profits at the expense of the environment. Nobody can afford wait three hours for a bus, especially if you have children or other responsibilities. The solution to wasteful transportation is not bicycles or other easy consumer-based decisions. We need governments to fund public transportation infrastructure. Etc, etc.
Thanks again!
About 8 years ago I did a rough estimate of my family's domestic production of CO2 via electricity, natural gas used for heating and cooking, and petrol for transport. Overall they came out as about equal in terms of CO2 produced.
Since that time I have sharply reduced the family consumption of electricity by replacing energy hungry gadgets and carefully avoiding unnecessary use. I have sharply reduced transport consumption by buying a super efficient diesel car and improving my driving style. I have added solar hot water and a small wood burning stove and a more efficient gas furnace and improved the insulation on my house, to reduce natural gas consumption, but the house is old, and there are limits to the improvements that are practical, or the lower temperature my family can tollerate in winter. Natural gas is now my largest source of CO2 emissions.
Of course, I also generate indirect emissions, both from the food I eat, and the goods I buy. I am vegetarian (but not vegan) and I grow a few vegetables in the yard, and keep chickens, which helps, and we tend to buy stuff second hand on ebay which reduces the embedded energy of our possesions a bit, but these numbers are hard to quantify.
Beyond that, there are the emissions triggered by the social environment funded by the taxes I pay. They are the hardest to evaluate or influence.
Technically yes, but forget about cups of tea and consider your time in the shower.
A gallon of water heated from 20C to 50C (less than half the temperature rise of Paul's example) is 30*4*3,785, or 454,200J. Factor in the same rough efficiency calculation Paul used and call it 50% of your wrong turn in the truck.
Most shower heads are in the 2-4 gallon per minute range, so one or two extra minutes in the shower roughly equals your wrong turn in the truck. Maybe three minutes if you have a low-flow shower head. A bit longer if you lower your water heater temp to something like 100F instead of 120F. How many of us take 7-10 minute showers when we really only need 3-4 minutes to get clean? I plead guilty.
At one point I realized that the carbon emissions from NG in our one-bedroom apartment were about half that of my daily 36 mile (roundtrip) commute. Almost all of that was from taking showers and washing dishes. Fortunately I'm no longer making that commute. Cutting down on the shower time after a hard day on a roof has proven more difficult.
Coming from an area of the world which should strictly speaking be barren desert were it not for Megawatts of water pumping, this article does lose a little bit of its impact. Glass houses, stones and all that.
"I am my master's dog at Kew, pray tell me sir, whose dog are you?" In other words, what kinda gigawatts are you using, strobe, to stay ahead of nature in your neck'a the woods?
Here we use it for AC, otherwise we would be sleeping in a pool of sweat like I used to do as a kid, and all of humanity east of the mississippi used to do- and I still do when the nights get sweltering.
Thanks, Tom, fur your as usual useful numbers, my wife liked it too, even tho it supported my little digs on her not covering the boiling pot.
It also supports my smug feeling about my $ 120 solar water heater, which in all the heat and swelter, gives us copious hot water for all purposes, including washing off all that sweat.
I once put in a solar hot water heater. It made a huge impact on electricity consumption (I know, electricity for water heating... I bought the house used). Probably one of the best things you can do to conserve energy. I would like to see them mandated in the new construction codes.
I have since moved, and may move again, otherwise I would put one into the current house. Portland Oregon area doesn't even get that much sun.
Jeff
Solar hot water heaters certainly should be mandatory here in Florida. You hardly need any roof space to get copious hot water (warm inlet water certainly doesn't hurt).
FWIW, tankless hot water heaters are NOT the answer IMO. I've had two for about five years (complicated why two) and I doubt they'll last past seven years. Rheem brand IIRC. I know it's just anecdote but I haven't seen my gas bills fall significantly since the remodel (electric oven and stove top so hot water is my only gas use).
A few random thoughts:
1. Natural gas transit isn't as efficient as most people think. I have a white paper somewhere on my hard drive that I'll have to dig up but losses from pumping gas the "last-mile" are rather large. Something on the order of 30%. @Tom Murphey perhaps this is worth investigating and integrating into your observations/calculations?
2. In areas where off-peak wind and nuclear is available, storing production as hot water is a sensible "smart- grid" application IMO.
3. Electric heating of all types seems like a sensible long-term infrastructure investment. Using wind, PV, nuclear, or (uhg...) coal to produce electricity frees up NG for transit either directly or indirectly via NGL which should buy us some time to transition to EVs and less transit- intensive lifestyles (perhaps some wishful thinking sprinkled in there).
TECO (Tampa Electric) has an optional 'special rate' tariff for high use electric applications in its residential market place - mostly water heating (and sometimes air conditioning). It requires the installation of a separate meter that is controlled by the utility so that during times of peak demand the consumer is shut off from the grid, lowering the total energy requirements the utility must fulfill. This lowers their marginal supply needs, and saves them money. In theory, it is supposed to save the consumer money as well. In practice, not so much.
I got much better results by installing a remote control on the water heater's electric supply line (X10 system) and mostly leave the heater shut off. Although it is not truly a 'tankless' system, I find it only takes about twenty minutes to raise the temperature sufficiently to wash dishes or take showers. I don't seem to have any problem planning that far ahead, now that I'm used to the idea. I set the interface with a macro that instructs the unit to switch off the heater every two hours, in case I forget to. Back when I had to take a shower every day, I had it switch on at 6:00 AM every morning as well.
I have never measured the savings in Kilowatts; but my electric bill is about $25-$30 per month less now - three times the savings of the 'off peak' meter system promoted by the utility.
A couple of caveats. The tank is relatively small - 30 gallons - which is why it recovers so quickly. When I lived in Vermont, CVPS supplied the water heater as well as the electricity, and they installed an 80 gallon monster that took a lot longer to heat up. Also, I keep the temperature of the tank set pretty high - 140 degrees F. You can have problems with bacteria - like Legionaires, for instance - if the water doesn't ever get above 110 or 120 degrees F.
And to end with a quibble, calling it a 'Hot Water Heater' is inaccurate as well as redundant. If the water was already hot, it wouldn't need to be heated, would it?
Hi Tom,
Great topic and great post. My drip coffee maker (Mr. Coffee) uses 180kJ to make a 400ml cup of coffee. I am not sure I want to calculate an efficiency for this because it does some local boiling to push the water up over the filter.
In Bob Everett's 2012 Energy Systems and Sustainability: Power for a Sustainable Future, the authors note that in the UK, electricity usage goes up by 1.6GW at the half-time of a World Cup game. Presumably a lot of electric tea pots. And probably with more zip than tea pots in the US because of the 240V supply.
Dave
It is great to see someone tackle the 'energy dashboard' for gas, even if only for a little while, and in an experimental set-up.
Based on measurements (electrical) at our house where my wife likes to cook, cooking energy is a small percentage of our household electricity usage, which in our case is small to begin with. I even discovered we could have an evening dinner party and handle the oven / range usage on our modest battery backed-up PV system during a power outage, after watching kitchen electricity usage for a few months using TED.
I would guess the optimum kitchen heating efficiency would be induction (which we have) using insulated, covered, low-mass, low heat capacity pots (which we don't have). You didn't assess what % of losses were due to heating the cooking containers, but induction could mostly avoid those with the right cookware, and avoids the significant blow-by losses with gas flames. Induction is the cooking performance of gas from a higher efficiency electrically fueled appliance. Our electricity comes from our PV system, though electricity production and consumption are often not in phase.
I used to vehemently dislike electric heat, but I've come to see that efficiently placed electric heat can be the 'least worst' option. Davis Energy Group once measured that 1/2 the hot water that left a residential water heater never came out of a faucet (half cooled in the piping network), so with a gas tank water heater efficiency at ~50%, the system efficiency was 25%, which is worse than a point of use instantaneous electric heater on a source energy basis. Low usage can be a real problem with centralized high efficiency hardware - efficient devices that aren't getting used or are connected to inefficient distribution networks often don't amount to good investments. Electric heat tends to be cheap to install, mostly maintenance-free, and easy to zone, so can make for a 'least-worst' choice in a low usage location.
As bad as your measured efficiencies seem, consider that they are probably much better and more convenient than what came before - most people seem to have forgotten that incandescent lamps were a substantial improvement over what they replaced...chopping, hauling, and burning wood to heat water makes your gas meter measurements look like a great deal, given the cost of your labor when at work.
My observation is that loads left running and forgotten are the ones to worry about - basement dehumidifiers, tank-style water heaters, old fridges, lighting in empty rooms, 'set it and forget it' thermostat operation with a tight heating vs cooling setpoint range...few people would leave their vehicle running in the driveway all day when they weren't using it, but think little of household loads that amount to the same thing. One predictor of someone's electricity usage is how many things they have running that they ignore or have forgotten about.
Well, since thermal machines are my hobby, I could not resist making a little wood water heater to fit in parallel or series with my solar heater, and I find on cloudy days an armload of any sort of wood trash I can pick up in the yard will take my 200 liter water storage tank to 50 C, which is enough for at least a couple of days of our usage.
Wood works well.
Dang it, I would love to give y'all a big report on my wood fired stirling PV backup generator but I just can't get that pellet gasifier working consistently, (koff, choke).
The neighbors would complain about all that stinky smoke, if I had any neighbors. Maybe tomorrow- if I live that long.
I found this story interesting as well - for different reasons. We also have a TED as well as a Kill-A-Watt, and it bugs my wife as it gives here the feeling that I am looking over her shoulders when she wants to bump down the temperature to run the AC. I guess what made it worse was when they added the ability to view the data online..
So I read the story to her last night just to prove to her that I could take this thing even further than I already have.
Our house has 5 appliances that connect to gas, and I *believe* that none of them have pilot lights.
* Gas clothes dryer.
* Gas fireplace (which has a pilot light, but we never use the thing and it is turned off).
* Tankless water heater.
* Gas cooktop (definitely has electric igniters).
* Gas furnace.
There were some repairs to the gas lines outdoors, and they needed to check all of our appliances before they would turn our service back on. I don't think there were any pilot lights that needed to be re-lit.
Was fascinating to read. I woulda thunk efficiency was more like 80%, so its pretty disappointing. On the microwave front, before installing ours last year I put in an the kill-o-watt. The nameplate power was 1200watts, but it consumes 1800. So if the nameplate number is correct the conversion to EM waves is about 67%.
I've been looking for a microwave oven recently. When the specs are read the power delivered is approximately 3/4 to 2/3 of the power used for most of the models I have looked at.
NAOM
No... No... Something else is going on there. The nameplate power is supposed to be the maximum power drawn by the appliance. The only clue as to the output power are the manufacturer's claims made on the cardboard box it came in or presented in some artwork on the front.
http://www.energysavers.gov/your_home/appliances/index.cfm/mytopic=10040/
Oh... You know... It may a problem with definitions. "Nameplate" is often used to refer to the small, drab, and artless block of text on the back of the machine:
http://www.jproc.ca/marconi/msl5_power_unit_nameplate.jpg
You'd have to know how the kill-o-watt deals with things like real vs. reactive power, power factor and harmonics, etc. Nothing's ever as simple as it seems, including measuring AC power draw!
The Kill-A-Watt measures true Watts, Volt-Amps, and presents the ratio (power factor). So it's doing the right math as an instantaneous current times voltage, integrated over the cycle. At least it's meant to be doing that. Can't vouch for how perfectly. But you can definitely see that incandescent lights, heating coils, etc. have PF = 1.00, while transformers and "fancy" electronics often have PF around 0.6 or so.
One quirk of the Kill-o-watt meter is when measuring usage from different inverters. Stepped ("quasi-sine wave") inverters will read lower on inductive loads than true sine wave inverters when measuring the same device (I think I got that right, don't quote me). Resistive loads, not so much. My most useful tool is my set of shunts and meters on the DC side of my system; measuring amps and volts. This allows me to account for differing efficiencies throughout the system. That said, the Kill-o-watt is a great tool, especially when doing comparisons over time.
Hoping to upgrade my older inverters soon.
Alas I cannot use the Kill-a-Watt meter to measure cumulative energy output of my inverter, since its output is intermittent (standby, load-sense). A mechanical KWH meter would have been useful, but harder to get. When the electrical company replaced my meter with a newer (but apparently just as primitive) one a couple of years back I tried to ask them to keep the old one, but they preferred to throw it away (I assume).
I've measured the percentage of the time the inverter output is "on" (i.e., that the frig and/or freezer are on) by plugging an electric clock (the simple mechanical type) into it. It does not draw enough by itself to be sensed as a load.
$25-$30 on e-bay, "watthour meter".
Meters are one of the most strictly accounted pieces of equipment at most utilities, the crew almost certainly had to return it to a clerk/accountant to log that serial number had been taken out of service, and re-issued or disposed of in an audited fashion.
On the plus side, a lot of tea is made in the winter when the lost heat is recovered as space heat.
Ah, but the utility of that depends where in the world you are. Here, in the tropics, just a bit of heat can make a room uncomfortably warm.
NAOM
Solution: Drink cool beverages in hot weather.
(And eat raw meat?)
I'll be taking care of that one later ;)
NAOM
I have a gas storage, water heater that can be used off a gas storage tank. I filled the tank with 200l of LPG but it was empty in less than a year with very little hot water used. I would estimate that it uses the 200l per year just to keep the water warm let alone heat it up to use. Needless to say, it has been a couple of years since I filled the gas tank and I just boil hot water to use as I need on the stove.
On the boiling pot, I made a steam generator, for the oven, using a large saucepan. I found that the heat lost through the sides cut the amount of steam drastically. The solution was to fit a loose skirt of foil around the saucepan, secured by a tight loop of wire at the top and a gap between it and the pan. The heat that spilled around the side of the base of the pan was trapped inside the foil. That made a large difference to the steam generated.
NAOM
I use a propane camping stove a lot. I use the little bottles for several reasons. It makes me quite aware of the costs. Making rice or hash-browns is expensive. Better coupling to the load can be had by making a tinfoil cone-collar about the pot and just as high that entrains the hot gasses rising from the burner... but it's a lot of bother.
I've lived with wood-stoves a lot. In a small, well-insulated space, two sticks burning, the smallest fire you can make, is plenty. In the cold of winter, making rice or hash-browns or long-simmered meats and vegetables in red curry is "free".
I've noticed that an induction hotplate will boil water faster than a microwave. Mine cost $99 at 99 Ranch Korean market. I see them for $60 at Walmart. It would be interesting to see the efficiency figures for that. There is a lot less wasted energy in an inductive hotplate: No heavy tube-filament loss and no core and wire losses within an under-rated transformer. The coupling to the water load in a shallow, flat, covered pan is very good, too.
Vacuum-insulated cookware systems allow the food to be heated to its cooking temperature and then transferred to an un-powered station to continue cooking using just the initial energy investment.
http://thermalcooker.wordpress.com/tag/cook-n-carry/
http://www.zojirushi.com/products/snxae
Manual PDF:
http://www.zojirushi.com/servicesupport/manuals/manual_pdf/sn_xae60_80.pdf
http://www.pinoyoutlet.com/image/cache/data/10-26-2011/Pinoy_021-500x500...
As a simple way to gauge their relative performance, I filled our electric kettle with 1.7 litres of room temperature water and plugged it into my Kill-A-Watt monitor. It shows that 0.16 kWh of electricity was required to bring this water from 19.6°C to a full rolling boil.
I then filled the smallest vessel I have that would accommodate this same volume of water and repeated our test using our portable induction hob (a Vollrath). To accomplish this same task with induction, 0.20 kWh were required. These values are approximate, but should serve as a general guide.
Our propane hobs are rated at 4.5 kW and my general impression is that boiling water with propane takes two to three times longer than with either induction or our electric kettle, and these counter top appliances draw just 1.3 kW each (~ 113-volts measured at plug). Needless to say, we never use propane except in the event of an extended power cut.
Cheers,
Paul
A very useful comparison: thanks! Either electric method is pretty darned comparable, compared to the much more wasteful gas approach. But I'll restate that the source of electricity is important. If simply from a heat engine, there goes most or all of the gain...
Your point is well taken, Tom. Our electricity in Nova Scotia is among the most carbon-intensive in all of Canada -- at 0.8 kg CO2(e) per kWh, it's four and a half times more intensive than that of Ontario and some four hundred times that of Québec ! Thus, boiling 1.7 litres of water in our electric kettle or on top of our induction unit would have a CO2(e) penalty of 0.13 and 0.16 kg respectively.
So how does that compare to propane? Using the widest flat bottom vessel that we have to ensure maximum heat transfer (22 cm wide), it takes approximately eight and a half minutes for our propane cook top to bring 1.7 litres of room temperature water to a full boil. As mentioned, our gas hobs are rated at 4.5 kW, so this translates to be roughly 0.64 kWh of propane demand. The CO2 emissions for propane are said to be 0.24 kg per kWh (source: http://www.engineeringtoolbox.com/co2-emission-fuels-d_1085.html), so boiling an equivalent amount of water using propane would result in the release of 0.15 kg of CO2; that's pretty much in line with these other two options, even though our electricity is more CO2 heavy than most.
For us, electricity is the clear winner. We subscribe to Bullfrog Power (http://www.bullfrogpower.com/), so all of the electricity that we consume is generated by wind and low-impact hydro (we purchase 1,000 kWh of green power each month, but our actual usage is now closer to 750 kWh).
For American readers, Jonathan M. Lee has put together a handy chart that shows the carbon intensity of electricity by state (based on 2008 EIA data); you can view this chart at: http://thepowerfactor.wordpress.com/2011/04/21/co2-emissions-per-electri...
Cheers,
Paul
I find it entertaining that TX, the king of fossil fuels, is in the middle of the pack, while DC is among the worst. Guess that says where gov't priorities REALLY are.
Well, there's some good news on this front (outside of the nation's capital where the situation appears to be hopeless). In 2008, 48.2 per cent of America's electricity was generated through the burning of coal and by 2010 that had fallen to 44.8 per cent. And as of April of this year, coal's share has dropped to 32 per cent, so the carbon intensity of US electricity is falling rapidly.
Cheers,
Paul
Yair . . . We have a Tefal Quick-Cup which just heats the water a cup at a time. It's a bit noisy but saves heating two cups if you only need one.
Cheers.
It looks to be a nice product. Breville makes something similar as well.
See: http://www.youtube.com/watch?v=qpH9UgCE7lE
I'm not sure if one-cup kettles are sold here in North America; if they are, they would be presumably much slower because our mains voltage is only half that of your own.
Cheers,
Paul
We do most of our cooking on propane, and my impression is that the propane used is negligible. I don't have a number for it, but I do see how much propane is used in total through the summer (about 50 gallons) for cooking AND to heat water for showers and laundry, and I think the latter is most of it, despite using an efficient on-demand tankless heater.
When I say "negligible" I am comparing with propane use in the winter for space heating. Despite using wood for half the heat. Of course, any propane used for cooking in the winter simply subtracts from the propane used directly for space heating.
That said, when the wood stove is in use we often cook on it. With the model stove we have, it'll bring things close to boil but not quite, good for slow cooking, or pre-heating a kettle.
OTOH during the hottest days in the summer we sometimes use an electric toaster-oven or crockpot outside to avoid adding heat to the house.
Hey, that looks mighty familiar!
Love, love, love my Vollrath induction hob.
That's great to hear. It's a solidly built product, very energy efficient and it runs circles around gas -- it's faster; you can set the precise temperature that you want to maintain and it will respond accordingly; there's no waste heat generated nor are there any combustion by-products; and it's easy to clean. If something spills over on our gas cooker, you're pulling apart and cleaning a multitude of grates, burner assemblies and so on; with this, you spray a little Windex and give it a quick wipe with a piece of paper towel and you're done. And if we want to keep the kitchen cool in the summer or if we're entertaining on the back patio, we bring it outside and plug it into a standard wall outlet.
We have two BergHOFF induction hobs as well, but the Vollrath is commercial grade and the difference in their performance and in the quality of their construction is clearly evident. Now that we have the Vollrath I wouldn't settle for anything less.
Cheers,
Paul
Great info on different cooking devices, and thanks to Wikipedia and you all, I now know what a 'hob' is.
Two overhead questions to all:
1. Are induction hobs/stovetops a big improvement over a resistive-heated glass cooktop?
This marketing video from Siemens makes the idea sound good...but are there any gotchas/drawbacks? At first blush it seems that quick food heating and perhaps lower electricity use sound attractive. Is purpose-made cookware required?
2. I am intrigued by the idea of pressure cooking, as a way to quickly prepare flavorful food, and this technique is more thrifty on energy than other methods, bonus deal.
I looked up this device, which appears to be made in Canada...no Teflon pot, stainless steel..good reviews...has anyone used this or one like it?
I came across this hunting around Amazon after trying unsuccessfully to navigate to the link to Paul's insulated slow cooker...
Under ideal conditions, a conventional smooth top electric is reportedly 55 per cent efficient in its heat transfer and a halogen electric could conceivably bump that up to 60 per cent. But pots and pans are not always properly sized in relation to that of the element, or they get bumped about in the cooking process or their bottoms don't sit in perfect contact with the element surface. In addition, a large amount of heat continues to radiate from the hob long after the pot or pan has been removed and the power switched off. I'm guessing real-world efficiency is probably closer to 35 or 40 per cent.
Induction hobs are typically 85 to 90 per cent efficient, you don't have to worry about the pot or pan being the wrong size or the bottom being uneven, the power draw is effectively zero the moment you take away the vessel, and there's no build-up of unwanted heat at the tail end.
In the video linked below, the author tells us that it took 0.191 kWh to bring a pint of water to boil using his conventional electric cook top and 0.071 kWh with his new induction hob. By his account, his induction hob is three times more efficient and five times faster.
See: www.youtube.com/watch?v=-OPk5sKyAgI
With regards to cookware, if a magnet sticks to the bottom of the pot or pan, you're golden.
Cheers,
Paul
Paul,
Thank you for the information...it is good to hear actual experiences to compare with the marketing material.
You're most welcome, H. As an added bonus, for every kWh of cooking related demand that you eliminate during the cooling season, you'll save an additional 0.3 kWh in air conditioning costs. And the savings can be even higher for those on TOU rates because we typically use our electric cookers during peak hours.
If we were to move to another home at some future date, I'd opt for the Bosch Avantixx which has something called Anti-Overflow Protection. If it senses that a pot is boiling over, it automatically cuts power to that hob. That's a nice plus in my books as I'm the one in our household responsible for cleaning up any mess.
See: www.youtube.com/watch?v=SJcU-U6BUAs
Cheers,
Paul
An electric kettle should be more or less 100% efficient at turning electricity into heat. Assuming that the container losses are approximately equal, and thus common factors that cancel, the efficiency of the induction cooker is ~80%. Which is to be expected from your average switch mode supply.
Our Vollrath is said to be 90 per cent efficient (source: http://www.vollrathco.com/catalog_product.jsp?id=5705).
Cheers,
Paul
What I find amazing, coming from the UK, is that you don't have a kettle!
This is an unimaginable state of affairs for me.
You could improve the efficiency of the gas burner substantially by making a metal ring about the same size as the pot which channels the heat upward rather than allowing it to flow out. I saw someone do this in an article somewhere, New Scientist possibly.
First thing: Pilotless gas appliances save a lot of gas over time, as your post shows.
Second: Actually bringing the water to a boil may skew the results a bit; sensible vs. latent heat and all that. It takes much more energy to push your water that last degree from not boiling to boiling than it did to raise it each degree before the boiling point.
Thirdly: Having solar water heaters and thermal storage sort of makes the efficiency thing moot. Our home-built system, solar collectors and an insulated, 1600 liter storage tank means that, over time, we have more hot water than we need. Since we also have an abundant supply of water (solar pumped to a tank), we can use as much as we want, within reason. A long, hot shower is 100% guilt-free (and essentially $$ free at this point) in our home. I'm sure that the overall efficiency of the system isn't very high, and don't care much. Normally, water at the kitchen tap (and dishwasher) is @60C as that leg comes off before the mixing valve. It doesn't take nearly as much propane to bring a pot to boiling. While we have a tankless backup water heater, it rarely gets used. Our winter hot water comes off the woodstove (basically reclaimed exhaust heat), supplemented by solar, again, not likely as 'efficient' as some of the new gas water heaters, and again, we don't care as it's essentially free at this point. Excess heat in winter gets dumped into the radiant floor.
As for the gas stove, I discovered long ago that covering a pot saves a lot of gas, especially when canning (need to keep several pots at boiling for hours), and reducing the flame size to the pot size also helps. There seems to be a point of diminishing return from cranking the burner on full. We use a small electric oven for baking smaller items (I can relate to your pizza dilemma). Since I usually bake bread and pizza on a stone, I generally sacrifice propane and realize the cost/benefit trade-off. A solar oven is in the works.
The gas (propane) clothes dryer is another matter (working on that too). Can't get the spouse to warm up to the solar/wind dryer thingy (clothes line).
Electrical efficiency is paramount in our home; PV with battery storage. My Kill-o-watt meter gets used alot ;-)
Thanks, Tom, for another useful and enjoyable post!
The problem with many (most?) of the current gas stoves is the oven. Yes, they use spark ignition for the cooktop but the oven runs a several hundred watt glowbar to keep the burner lit. It's on whenever the burner is called for.
I'm lucky as far as the dryer/clothesline question, my wife avoids using the dryer whenever possible, she'll hang clothes up indoors before she'll use the dryer! At most it sometimes gets used to fluff (no heat) the clothes after they've dried.
A Kill-O-Watt meter is a good friend.
I'm toying with the idea of putting a hot air box on the roof just above the dryer to heat/preheat the inlet air to the dryer. On hot days, the dryer could be run on the no heat setting. Just another project in a long list.
...and yes, our gas over has the glow bar thing. Kill-o-watt reports about 140 watts when it's on. I hope to replace the whole unit with one that doesn't plug in; hard to find, especially one without a pilot. We may be converting to wood anyway, depending on how things play out.
On a hot day, why don't you just hang them up outside?
EDIT: didn't read your first post properly, why won't they want them hung up outside on a hot day? What's the objection mostly?
From my comment, above: "Can't get the spouse to warm up to the solar/wind dryer thingy (clothes line)."
Posting past each other ;-) It's complicated... [thinks about sabotaging the dryer for a while]
My wife has been very patient regarding my 'eccentricities' to this point. It's been remarkable that she (a fairly coddled Jewish city girl) has adapted so well to living off grid. She's even embraced the occasional TOU requirements. Since most of my contraptions actually work (with little user participation required), I'm doing ok. If I could build a clothesline that hangs the laundry, drys it and folds it, she would love that ;-/
Going off grid is easy; doing it 'seamlessly' is a bit trickier. Most visitors never realize we're not connected to their power grid.
Er ... I am not really the man to suggest this (and I am in awe of you and your family achievments, and domestic tact) but I will anyway ;)
Could you do the clothesline and folding bit more often?
We use a spindryer (oldfashioned) to squeeze moisture and spare the washing machine high speed stress and I do watch the weather and bring in the washing on occasion. We do hang fairly dry washing 'to air' overnight indoors especially in the winter: and other ad hoc 'temporary' domestic delights until I get round to fixing a better system.
Yeah, Phil, one way we preserve domestic tranquility is to do our own dirty laundry ;-) I do my nasty work clothes, she does her stuff; avoids several pitfalls... and I do dry some of my stuff on the line, though underwear and socks can be a bit stiff if not tumbled for a bit.
I'm interested in finding an old washer that could be driven directly from a windmill, maybe one of the old pulsating type, with the roller/wringer. The ones I've found so far are priced as antiques. Maybe as sellers get more desperate...
Another option would be an all-in-one unit with the heatpump dryer ($$!).
Ghung - What a flash back! I hadn't thought about it in many decades but we had a hand cranked washing machine when I was small child. The amazing thing must have been the gearing. You cranked constantly in one direction but the blades inside alternated back and forth. One problem was putting in large sheets and such. They would occasonaly get stuck under the blades. Remembering watching grandpa take it apart to free them. Prior to that I remember grandma and that wash board on the back porch. And that was before we had our first inside toilet. And no...not a country boy. Grew up in the inner city of Nawlins.
Was curious and found this: http://www.cleanairgardening.com/portable-washing-machine.html Looks too small
But found this one. http://www.youtube.com/watch?v=7N-4-3Qb2gY Looks kinda familar but it seems ours was more vertical. IOW blades on a vertical axle.
Yair . . . We have had one of these for close on forty years. They were available in 24v, 32v, and 240 mains.
http://www.google.com.au/imgres?q=lightburn+washing+machines&um=1&hl=en&...
Cheers.
Wow, Rock, the first link looks like more trouble than it would be worth. I guess it's all relative. One I'm looking for looks more like this one.
I have a feeling we'll end up with one of these, very efficient, and cheaper than the antique.
"I have a feeling we'll end up with one of these": Ventless Front Load Washer Dryer Combo
There is a ventless dryer here. It collects water, yes, but also blows hot wet air out the bottom. It takes forever to dry anything. It has broken twice with long waits for warranty service. The matching high-efficiency washer is a pachinko-machine with lots of lights and sounds. It takes forever to do a mediocre job. The wrong soap or too-much of the right soap causes flooding. Its predecessor ate its main bearing within a few months. From watching all of this, the observation is: simpler is better.
I have to assume you're familiar with Lehman's Catalog. They are Amish, or originally so, and offer a lot of hand-driven home tools/accoutrements..
http://www.lehmans.com/store/USA_Made___Home___Lehman_s__Laundry_Hand_Wa...
$500/$650 with or w/o hand wringer..
http://www.lehmans.com/store/Home_Goods___Laundry___Washing___Galvanized...
and curiousity also got me googling for vids of Pedal Launderers..
http://www.youtube.com/watch?v=KzTKDw-b9uo (this one is in South Carolina!)
and here is one direcctly adapted from existing Washing Machines..
http://www.youtube.com/watch?v=_7XHItI0dA8&feature=related
and THIS one looks almost serious.. 55 gal drum, and the guy is reading a book as he pedals away on a big load!
http://www.youtube.com/watch?v=ymCvPOq4Tlc&feature=related
and Finally, I like the 'making of' details in this one, including the early failures and commentary..
http://www.youtube.com/watch?v=ymCvPOq4Tlc&feature=related
I got one of those. It's not all that sturdy, the plastic knob on the top didn't last long. The rack its setting in isn't much either (and mine looks sturdier than that one) and gave way after a couple of years. I dropped and cracked it last year, still works pretty good I just can't put hot water in it. It arguably works better with out the rack, you put in a half dozen socks or a couple of shirts and give it a good vigorous shaking for about 60 seconds. It is small but its quick on lightweight stuff, like when you are going to bed and realize you don't have any clean underwear for tomorrow.
A couple of years back I replaced the kitchen range (propane fueled) with the only brand I could find (in the USA) that has the following features:
* no pilot light (electric spark ignition)
* no glow heaters in the oven
* can start the _oven_ (not just the top burners) with a match if the grid is down
Something that I have been thinking of doing with a drier is to recover heat with a recuperator. Run the exhaust through a bed of something that will absorb heat and then run the inlet air through a heated bed the opposite direction. It would probably work best in the winter time. You would need to switch back and forth between the beds. You could do it just on time, you don't need temperature controls on it.
The way recuperators work is that the hot gas heats up the material and then passes on through. There is what is called a “heat transfer zone”, where the solid is heating up and the gas is cooling down. That “heat transfer zone” propagates through the bed with essentially isothermal zones on each end, one heated and one cold. When you put hot air in, the heat transfer zone goes in one direction, when you reverse flow it moves in the opposite direction.
A lot of the heat load in a drier is the evaporation of water. You don't get that back unless that water condenses. You can get some of that in winter. In summer all you are going to get is the sensible heat of the exhaust which isn't that much, but it is better than nothing. You need the beds to be low pressure drop which means pretty large diameter. Hot gas flowing down gives you stratification stability so the flow is counter current.
That kind of regenerator is often made with checkerwork. Cinder-blocks are the easiest to find and stack. The pieces of smashed sinks or toilets are much finer grain along the edges and have sealed flat surfaces. Since you don't need it to be truly refractory and don't want it to be porous or reactive, nor to out-gas or retain lint, something like bundles of stainless-steel pipe also suggest themselves.
That last degree doesn't cost much more than the one before it, but then things change radically. At 2257 kJ/kg latent heat, vs. 4.184 kJ/kg/K to heat the water, evaporation is very costly. Heating water 80 °C costs 335 kJ/kg, but boiling it all off takes 2257/335=6.7 times as much. This is why the water will remain at boiling as energy continues to be dumped in, rather than flash into vapor right at the moment you reach 100 °C. But I'm likely not telling you anything new.
The vapor pressure does increase as the temperature increases:
So increasing amounts of energy can be piped into evaporation as the water heats (you see it rising from the pot). But the last degree isn't particularly special.
Putting a lid on it recovers some of the heat of vaporization, and delivers the (still pretty hot) water back to the pot.
As a toy example, a lidded pot containing one liter of water and one liter of gaseous space above will be able to support about one gram of water vapor at full-saturation, coming at a total evaporation cost of 2257 J. The incremental cost of going from 99 °C to 100 °C will cost 4184 J in heating the water, while going from 733 to 760 mmHg of water vapor (steam) costs 3.5% of the total evaporation budget, or an extra 80 J. I have not fairly accounted for losses of water vapor out of the cracks around the lid, but the point is clear enough that even in the last degree where the rise in vapor pressure is steepest, the energy cost for evaporation is not competitive with the straight-up heating of the water.
Yeah, Tom, I actually used "that last degree" to simplify things, understanding that latent heat is required for phase change while the temperature remains constant.
I was trying to explain to a canning group recently how water can reach boiling temperature without actually boiling (while trying to explain the advantages of pressure canning). I eventually gave up; figured it's better they understand the rules of canning even if they don't get the physics. One woman simply couldn't understand how water will never exceed boiling temperature (about 208/98 at our altitude) at atmospheric pressure, no matter how hard she boiled it (which is why we use pressure canners).
BTW: I got most of my useful education in thermodynamics in the Navy (thanks to your tax dollars); steam turbines, superheated steam vs. saturated, all that. It stuck with me much better than my college courses on the subject. Here's to applied physics ;-)
Tom, thanks for explaining how lidding the pot works, it was a "click" as the light went on.
NAOM
Fantastic post. Thank you.
Regarding latent heat, it would be super neat to weigh the water to the 10th or 100th of a gram before and after "boiling." Then the latent heat loss could be calculated and the efficiency adjusted accordingly for when everyone boils water in a pressure vessel like civilized folk.
Regarding latent heat it might be neat to use a block of steel of known weight as a non-latent-heat affected benchmark.
Tea does not need boiling water and it is unfortunate that so many appliances have such fantastically poor temperature controls they rely on boiling for the cutoff.
What about the energy efficiency of using the laser pointer or electric meter for boiling water directly (not serious)?
The pointer seems to be a fantastic and non-invasive way to get data from obtuse things such as gas meter -well done.
You would need different kettles for different altitudes if you only relied on temperature. Would a temperature based kettle switch off if a low pressure system went through?
NAOM
My understanding is that the lid is mainly to stop convection. With a lid, the air inside the pot rapidly saturates and, once the lid and walls are warmed to the temperature of the liquid, evaporation stops.
The cracks in the lid are insignificant so long as the vapor pressure of the liquid is less than the atmospheric pressure, as some N2(g)/O2(g) escapes from the vessel to make space for the increasing concentrations of H20(g) (exceeding atmospheric pressure would require a pressure vessel, such as a pressure cooker).
Without a lid, the light air rises and is replaced by dry, cold air that allows more evaporation to occur.
Considering that it takes roughly a 'medium' flame to keep an uncovered pot at boiling, the losses are pretty substantial.
Thanks very much for this, the results are somewhat startlingly low, which has most of us looking for possible sources of error.
Experimental design: Seems like it might be informative to weigh the pot of water before and after to determine heat of vaporization losses. Also, as to maximizing efficiency on a stove top without a skirt on the pot, when I was a wee boy my Mom taught me to tune the flame to the bottom of the pot (get as close as possible to putting the inner cone of the flame at the contact point), and my high school chem teacher told me the same thing about bunsen burners.
One of the other posters mentioned gas quality and meter error. If you were using the same gas supply and meter when doing the tankless test, I'd be inclined to discount that given the high result obtained, but if not, it might be a good idea to replicate one test at both houses to compare meters.
On the storage water heater test, the temperature of the water in a storage tank is not uniform. Did you only spot check outlet temperature, or monitor thruout withdrawal?
This post helps us to understand the energy savings of eating raw foods as opposed to eating cooked foods. As an added benefit, raw foods (fruits and vegetables and sprouted grains) are much healthier for you. When eating a raw food diet, there is no reason to use the oven or stove. Also, not only does consuming a diet rich in raw foods avoid the use of energy in the home, it also is inherently lower energy consuming when sourcing the food.
"...it also is inherently lower energy consuming when sourcing the food."
That depends on a lot of things; location, climate, season, etc.. How many folks can eat a balanced raw diet year-round without importing foods log distance at least some of the time?
Ever visited Canada? In winter?
Cheers,
Paul
The Inuit eat raw fish and blubber ;-) (and what's that stuff they bury in the ground to rot for months?)
Perhaps I'm getting too soft in my old age, but seal flippers aged in rotting blubber doesn't carry quite the same caché as it once did. :-)
Here's to Hamburger Helper !
Cheers,
Paul
High in Vitamin C! "Wherever collagen's made, you can expect vitamin C." Weight-for-weight, raw whale blubber is as good as orange juice. Who knew?!
The Inuit Paradox: How can people who gorge on fat and rarely see a vegetable be healthier than we are?
http://courses.washington.edu/bioa101/articles/article41.pdf
Yummmmm, spicy pickled cabbage or kimchi.
I think they just call it "stink fish". The conventional belief is that white people can't eat it.
While there is some energy savings to be had by not cooking with gas or electricity on a raw diet, it's not as though people that practice a raw diet don't use any electricity for food preparation. Dehydrators, blenders, juicers and food processors are all normally used by those who practice a raw diet. The bigger energy savings occurs from not eating animal products, which are incredibly resource intensive.
I'm definitely on board with with a a mostly or even 100% plant-based diet. A raw diet may have it's benefits, but getting people to reduce the amount of animal products they use is difficult enough. Trying to convince people that not only should they only eat plants, but then eat those plants without cooking is a tough sell.
Grazing in the garden is about as energy efficient as it gets. I'll eat raw beans, squash, tomatoes, etc, as I pick, sometimes avoiding a meal at the house. We're also planning a solar dehydrator in the garden.
pre - But you can keep energy consumption down while cooking if you have the right equipment. I finally convinced my wife she wouldn't blow herslef up and got her using a pressure cooker. She can't get over how much it has cut cooking time/energy comsumption. On top of the PC she already had an induction stove top. For instance took her 20 minutes on stove top time to cook a pot of yummy beans instead of a couple of hours. I know she'll never admit it but I'm sure she hid in another room the first time she used the PC. LOL.
Humans can't really survive on a completely raw diet. There are no societies that ever have not used fire for cooking. "Hunting and gathering" in a supermarket in a city and people on a 100% raw diet lose weight and women become amenorrheic. A completely raw diet isn't something that can be sustained.
Cooking greatly increases the digestibility of essentially all foods. You have to eat more food if you don't cook it. Cooking food saves energy by reducing the amount of food you need.
Your assertion that people cannot live healthy on a rawfood diet is a myth. There is a whole subculture of people who live exclusively on raw foods. People do loose weight as they revert to a healthy weight, instead of being oveweight. This is because raw foods contain a higher amount of nutrition (vitamins, minerals and enzymes) which allows a person to live healthy on fewer calories. Cooking whole foods is still a lot better than the plethora of processed foods which pervades our grocery stores
I don't eat a diet of 100% of raw foods, my diet is about 60- 70% raw. I know of people who have lived on a raw food diet for years at a time, and some live exclusively on a raw food diet. They clearly are healthy. I am clearly healthy. I am 54, have litttle grey hair and have all of it, and weigh 162 pounds on a 5 foot 10 inch frame.
The problem is that our society has gone way too far towards eating processed and cooked foods so that people don't think of it as an option.
Eating raw foods in the wintertime is challenging. Importing foods is usually necessary. But some raw foods do store well. Apples, carrots and beets store well if kept cool (just above freezing). Some dried foods store well such as nuts, and others can be sprouted - I routinely sprout lentils. Also, as another person pointed out, there is a whole category of fermented foods which store well for long periods.
This is a fun article. Thanks for making these measurements.
In the pursuit of energy efficiency, cooking is a low-hanging fruit. The basic design of pots and pans is flexible, but prehistoric: "put bucket of food on fire." Seems we could do better than that.
The camping supply companies have been working on heat transfer efficiency. Like NAOM, MSR has made a heat exchanger that they claim improves the heat transfer efficiency from a gas stove by 25%.


They also make a cook pot with a built in shield that presumably holds hot gases near the pot for a longer amount of time.
It might be fun to test one of these. . . and then work out their embodied energy . . and figure out how many meals you have to make to end up saving energy over your existing cookware. . .
As augjohnson pointed out, nearly all modern gas ovens use a glow bar which is on the entire time the oven is on. I measured ours at 300W. It is more durable than spark ignition but definitely more wasteful. I have found only two companies making ovens with pilot or spark ignition - Premier and Summit Appliance. If the power goes out but you have gas and matches, it would be nice to still be able to use the oven.
Also, once you get a pot hot there shouldn't be a reason to keep the fire going, you just need the food to stay hot for awhile. Hayboxes were designed long ago for this purpose. The modern vacuum cook pot kits from Japan (that KalimankuDenku mentioned above) are descendants of the haybox.
And finally, since we're having record high temperatures, we might as well fry an egg on the sidewalk. We've been using the inexpensive Sport solar oven at our house and it works fine as long as we start the cooking before noon and the sky doesn't cloud up too much.

There are some very efficient camping kettles that have a central vent surrounded with a water jacket. These use very little fuel and arn't affected by the wind.
It would be interesting to see how efficient they would be on a gas hob.
http://en.wikipedia.org/wiki/Kelly_Kettle
These are cool!
The haybox is very interesting. The closest I've gotten to this is annealing steel in layers of fiberglass cloth or cooking MREs in my folded-up coat.
Chambers stove
http://en.wikipedia.org/wiki/Chambers_stove
This is a highly insulated oven:
"Chambers' patented method of manufacture used thick rock wool insulation to insulate the oven on all sides. This made it possible for the heat inside the oven to build up over a short period of time. The gas was then turned off, causing a series of internal dampers to close, which effectively isolated the oven compartment from the outside air. The food would continue to cook on retained heat, thus conserving fuel and reducing food shrinkage. This method of cooking, Chambers literature often claimed, also increased the food value of the cooked items."
An electric resistive or induction one would be interesting.
If you want to try something fun with solar, make a cone of metalized Mylar about two feet long that starts at ten inches in diameter or so and narrows to a point while having the metalize on the innermost surface. When pointed at the bright desert sun, the point will ignite with a "pop". Such non-imaging optics could direct sunlight into a highly insulated oven through such a small hole that the convective losses would be greatly reduced through it.
Highly insulated refrigerators have been mulled-over in these discussions. I make ice-boxes with R-30, R-40 insulation or better... insulation up to and beyond eight inches thick. The ice behaves quite differently in that cubed ice will solidify into a block of similar packing density. The ice must be out of the water and supported on a smooth, non-textured shelf.
The low tech way to do that is to drop the parts into a big bucket of wood ashes. Keeps the metal hot for an amazingly long time. I used the same bucket of ashes for five years, and only had to top it up a couple times.
Ahhh!
On the topic of ovens, we're forgetting, or at least I did, that Tom found that the majority of the energy required went into heating up the oven. So insulating an oven helps some but there still is the investment of energy required to get it hot. In order to reduce wasted heat perhaps the major changes we need to make are to our habits. The key trick is probably to avoid baking but when you do turn the oven on, bake a lot of stuff. And avoid heating water, but when you do heat water using fossil fuels, only heat up the amount you need and no more.
Chances are our grandparents, or their grandparents, followed these guidelines as a matter of course.
We can cook just about everything we like in our Breville Smart Oven (http://www.breville.ca/cooking-1/the-smart-oven-tm.html). I can't even recall the last time we turned on our conventional oven.
However, for maximum energy savings, it's hard to beat a slow cooker. Ours draws 163-watts and so five hours of cooking time works out to be 0.82 kWh. Actually, it's less than this because the element will start to cycle on and off once it's up to temperature and we can turn it off before the meal is done because there's enough residual heat left in the ceramic pot to continue the cooking process for another half hour or more.
By comparison, our convection oven draws 5.2 kW and takes approximately ten minutes to come up to temperature (5.2 kW x 0.17 hrs = 0.87 kWh), and so it has already scarfed-back more energy than our slow cooker even before we get things under way.
Cheers,
Paul
Where did you get that cooker? The only ones I've ever seen are totally uninsulated. That looks like it's got insulation around the heating element and pot. I want one!
It's a Toastess MultiPot MSC569 and was purchased at Sears.
See: http://www.toastess.com/products/view/slow-cookers/MSC569
Much better insulated that its predecessor, and I place a folded towel over the glass top and wrap a second towel around the sides to further reduce heat loss.
It was originally priced at $59.95, but Sears no longer sells this model and it was open stock, and so it had been marked down to something like $24.95. My partner is a retired Sears executive and so we received another 25 per cent off that by way of his employee discount, which meant our final cost was $18.00 or $19.00 CDN.
Cheers,
Paul
Thanks very much!
You are always a wealth of information! This is the sort of device I've been looking for.
My son did a science fair project comparing energy used to heat a few cans of beans using various appliances, and I was surprised that the crock pot (which took FOREVER to heat it) was among the worst. It had a lot of mass and not much insulation.
The invention I want to create is a super-insulated pressure cooker, like your unit only pressurized, so as to raise the temps a bit to tenderize more quickly.
I can believe that Crock-pots are bad for Electric Use.. yet they are really good for scheduled cooking.
While Reading this post, I was muttering 'Solar, solar, solar!' .. knowing that transfer efficiency is far less worrisome with a free and abundant source. (Sorry PNW and Scotland, YMMV of course)
I've long chewed over the idea of a Solar Crockpot that included some storage and managed dispersal of heat, so it can persevere on partly cloudy days. These would also generally need to have a backup source, electric or gas.. but still could mean great savings in energy purchased.. and lots of meals prepped at Bedtime or Breakfast, and hot and ready by Dinner, even when noone is home through the afternoon!
Hey Bob,
Depending upon the task at hand, slow cookers can be good performers provided they're reasonably well insulated (most aren't) and/or you take steps to reduce their heat loss, perhaps along the lines of what I've described above. And, as you can appreciate, certain foods must be cooked/maintained within a recommended temperature band to ensure safe handling, so unattended cooking in our Maritime climate could prove a tad tricky.
The first meal that we prepared in our slow cooker as I recall were four boneless chicken breasts and accompanying vegetables, and I don't believe that this required any more than 0.5 or 0.6 kWh in total. I posted this in another discussion about cooking appliances a year or two ago, so I'll see if I can locate that post and get back to you with the particulars.
Edit: OK, found it.... as posted August 15th, 2011:
Cheers,
Paul
Good points, Paul.
I agree that these would need to be well insulated, and why not, of course.. but particularly with a fickle Sun, you want to keep your temps where you need them.. and as above, I'd have a resistive coil still in place as well just in case, so it can pick up if the day goes grisly, or even a couple clouds hang overhead just a bit too long.
Turns out this something like what I'd envisioned already.
http://instantpot.com/technology/three-generations-of-electric-pressure-...
Probably still not terribly efficient, but at least the manufacturer considers energy consumption as a feature.
These particular units have a much higher power rating for more rapid cooking, apparently including browning. Think I may get one, put it on the Kill-A-Watt, and see how it works.
Thanks for the link to the instantpot. I'll have to take a closer look at this product.
I confess I'm not altogether sold on the technology. My parents had a Presto brand stove top pressure cooker, circa 1950, and used it regularly. It was fast and efficient, but everything came out tasting bland and mushy, with a texture and consistency not unlike that of baby food. It may have been improperly used, but I haven't been able to shake-off those childhood memories.
Cheers,
Paul
I have a one-pot meal that I've made for years. In the bottom of the pot go onions: these are your blast-shield... you can do terrible things to onions and they just caramelize. Then carrots, celery, turnip... the hard vegetables. Then the softer vegetables that will cook with less heat. Then the meats - perhaps pre-fried if it's pork. Then a layer of cabbage, sometimes a disc of cabbage sliced from the head, that acts as a support for the last feature, rice, and keeps it from swimming in or through the stock. Some stock or curry is added and the rest of the water will come from the vegetables, especially things like tomatoes or cucumber. It is covered and brought to a low boil for just a bit and then turned down to a very low simmer, just barely, for maybe forty minutes. When everything goes right, there is lots of flavor.
This is all preamble to saying: If the pot is allowed to boil late in the game, the flavor is gone. I imagine that is what's happening in the pressure cooker.
Another thing that kills flavor is apple. If a dish has gotten out of control with seasoning, adding apple will start turning it into water. Apple sauce can be served with a dish that is too hot for some guests. Rice or potatoes also attenuate flavor. The lazy dish above is better if the rice or potatoes are made separately. It is also better with layered flavors, like sauteed-onion + raw onion in at the beginning + onion added later + green onion garnish at the end or chilli paste that's been fried + chillies at the beginning + hot sauce at the end or veggies fried + veggies not fried in at the beginning.
Thanks for the tip. The Instant Pot is reasonably priced ($129.95 CDN, including shipping), so it wouldn't hurt to try one out and if for whatever reason we can't put it to good use we can always pass it on to someone who will.
Cheers,
Paul
The reviews of the Instapot electric pressure cooker were majority very favorable.
I particularly liked the review stating that a guy thew in some /frozen/ chicken breasts and certain spicy seasonings, and was rewarded with spicy-flavor-infused chicken breast that one could cut with a fork.
Other folks asserted that the short-time pressure cook was favorable towards keeping more of the nutrients intact in the veggies, vice long slow crock pot cooking.
Others asserted that the electric pressure cooker afforded much finer control over the process than the old pressure cookers that say on the hob...the ones with the rotating sputtering pressure-relief valve.
The reviews lead me to believe that it is important not to overt-time/over-cook...the device is reported to cook food /fast/
When it comes to cooking technology I prefer the other extreme - a pressure cooker. The lid is clipped on air tight witha rubber seal, and a weighted release valve keeps the steam in up to about 2 atmospheres pressure. Cooking time is reduced by about half. For white rice, once the vessel is up to pressure, I cut the heat and in ten minutes or less the rice is cooked.
You have to be careful not to overcook the food.
I was surprised to find out that my oven did not use a thermostat to regulate the temperature but balanced the level of flame against het loss through the uninsulated back! A couple of meters of fibreglass later and I am using a LOT less gas for baking. The back was insulated and remaining glass went into the top, below the burners, resulting in a much cooler oven top too.
NAOM
Question, what pressure is that at?
Also If that's for pure NG, I wonder how pure the NG delivered into our homes is. That might explain some of the apparent inefficiency.
Amounts are usually quoted at atmospheric pressure.
As far as gas purity: while not pristine, it's not dirty enough to make that much of a difference. Average composition in US homes:
So, about 2.8% of stuff that won't burn.
(source)
I drink tea throughout the day. The minimum level of water in the electric kettle is about 2.5 cups, so there are 1.5 cups of boiling water left in the kettle which waste heat by cooling down before my next cuppa.
To minimise the heat loss, I top up with cold water straight after pouring out the hot. A large cool mass loses less heat than a small hot mass. It makes quite a difference, judging by the shorter time required to heat to boiling again.
I boil a full kettle first thing in the morning and pour the contents into a thermal carafe, throw-in a couple tea bags and we're basically good for the day. I refill the kettle immediately after use to take advantage of any residual heat and it sits on the counter until the next morning by which time the water has come up to room temperature.
Cheers,
Paul
You win. But you don't drink tea, you drink stewed teabags.
It may not be to everyone's liking, but I'm OK with it. Your best option would be to replace your kettle with one that has its element built into the base.
Cheers,
Paul
This just in...
A neighbor of mine learned of the Do the Math blog, and invited me to test the efficiency of heating water via his tankless system. So we did the test this morning.
In a 2 minute trial, we used 20.7 liters of water, heated from 26°C to 47°C (1.82 MJ), meanwhile watching the 0.5 cf dial go around 4.18 times for an outlay of 2.25 MJ.
Answer: 81% efficient. I'm impressed. The exhaust is cool enough to allow the use of a PVC pipe, which is a reflection of the unit's efficiency: getting a good deal of the heat into the water.
Some of the 19% loss will be in the delivery system (pipes radiating, etc.), but the hot water discharge we tested was just on the other side of the wall from the heater—so not much obvious loss there.
Our Bosch tankless works similarly; the exhaust pipe is cool enough to keep your hand on. One caveat: depending on water quality, they need descaling occasionally. They make a connection device to allow easy connection of a small pump. White vinegar works well.
I suppose any gas water heater could benefit from an annual descaling, especially in hard water areas. Electrics; just replace the heating elements every few years. Also, drain and flush. The need for this sort of maintenance is generally ignored until something goes wrong. No telling how much I've saved folks on energy and $$ just by cleaning their air conditioning coils.
Another efficiency check is to ensure that gas/propane pressure is within spec to appliances.
Ensure attic ventilation is clear and working... vent fans.... crawl space vents... dryer vents; check 'em, clean 'em. Out of sight, out of mind, but in your wallet.
Good advice. Also, for tank-type water heaters, the trick to keeping them alive is to replace the sacrifical anode every five years or so.
http://www.hot-water-heaters-reviews.com/anode-rod.html
Thanks - great post. I have been blogging for a while about getting New Zealand to achieve 100% renewable electricity. One of my key arguments being that using natural gas to generate electricity at 35% thermal efficiency is dumb (especially for NZ as we could so very very easily and simply get to 100% renewables) and we would be much better off as a country to save our gas for direct heating domestic and industrial applications - after all, my argument goes, we are an isolated nation and are going to look extremely stupid if we have to build a pricey LNG import terminal because we have squandered what little gas we have on electricity generation. Suffice to say, this line of thought depends heavily on the general efficiency figure one assumes for direct water heating applications and as your numbers don't reinforce my worldview strongly and I don't want to "re-adjust my intuition about how straightforward it is to channel heat from a flame into water on the other side of a metal wall" , I am going to call you out a little.
So while I commend you for actually doing an experiment rather than just clicking around in cyber space looking for nice figures like I do, and forgetting your stovetop heating figures for one minute, I need to do a double take on the 64% tank heating efficiency figure you have come up with. Many manufacturers of instantaneous gas water heaters claim a 95% thermal transfer efficiency (http://www.rinnai.co.nz/waterheating_range.html?&subcategory1=41), and while this might be a stretch, it is a long way above the 64% figure you infer is a maximum / typical figure. 86% maximum is noted in Wikipedia as a maximum and this intuitively seems right to me, after all, I can't see how flu losses (primarily) could be as high as 36% as a general rule after separating out the tank and transfer losses as you have elegantly done. This paper here is interesting (http://cibo.org/pubs/whitepaper1.pdf) and suggests that 75% is the efficiency figure to use for new industrial type boilers.
"Gas fired [water] heaters have maximum recovery efficiencies of only about 86% (the remaining heat is lost with the flue gasses)" [wikipedia]
So I am going to go out on a limb and suggest a few possibilities as to why your figure is too low for me:
A - your gas meter is out of calibration a little - while your clever laser system got around the poor output readability, it does not check the accuracy of the gas flow volume.
B - you are also getting ripped off with regards gas "quality". You suggest a figure of 1020BTU/cf however this can vary substantially with natural gas depending on the methane content.
C - your water heating system tank is of poor design (how old is it? Is it insulated well? You say the flu gets very hot so it must be rubbish)
D - there is something more complicated going on in terms of thermal layering and heat distribution in your hot water tank - I suggest you need to repeat your experiment by emptying the entire tank.
E - All of the above.
Keep the interesting posts coming. As for me, your figures are politically unacceptable and I am going to go with 75% from now on.
http://freenrg4nz.wordpress.com/