Goodbye Helium, Goodbye Brainscans
Posted by Rembrandt on January 17, 2008 - 11:00am in The Oil Drum: Europe
 Some of the great things that make human live much easier are dependent on rare non-renewable resources. Helium is one of these, a noble gas with remarkable qualities due to its inert state. It is used for example to cool metals needed to create superconductivity. This process is applied in the medical industry to make Magnetic-Resonating-Image( MRI) scans, a technique to produce images of body tissue, making accurate diagnosis of health problems without surgery possible. But Helium is also applied in nuclear magnetic resonance spectroscopty (NMR), for the arc welding of various metals amongst which are titanium, magnesium and aluminium, to reduce high-pressure risk in deep-sea breathing systems, to purge and pressurize liquid-hydrogen rocket propulsion systems, to find leaks in pipelines, as a coolant in certain nuclear reactor types, possibly for superfluid gyroscopes and last and for me definetly the least, to let balloons float.
Some of the great things that make human live much easier are dependent on rare non-renewable resources. Helium is one of these, a noble gas with remarkable qualities due to its inert state. It is used for example to cool metals needed to create superconductivity. This process is applied in the medical industry to make Magnetic-Resonating-Image( MRI) scans, a technique to produce images of body tissue, making accurate diagnosis of health problems without surgery possible. But Helium is also applied in nuclear magnetic resonance spectroscopty (NMR), for the arc welding of various metals amongst which are titanium, magnesium and aluminium, to reduce high-pressure risk in deep-sea breathing systems, to purge and pressurize liquid-hydrogen rocket propulsion systems, to find leaks in pipelines, as a coolant in certain nuclear reactor types, possibly for superfluid gyroscopes and last and for me definetly the least, to let balloons float.
Can Helium be substituted? The answer is no for applications which need cooling below a temperature of minus 210 degrees centigrade since that is the temperature at which the next best thing, liquid nitrogen, freezes. Helium on the other hand only liquifies at minus 272 degrees centigrade and stays in that state even down to absolute zero. Making it the most precious element for cooling at very low temperatures. For MRI scanning this means the available substitutes can only offer much higher temperatures at which the scanner can operate, implying less conductivity and therefore a less effective scanner.
The availability of Helium is thus quite important as long as no substitutes for these processes have been developed. So how long will this resource last?
Introduction
Helium is a gas that over time came into existence mainly from the radiogenic decay of uranium and thorium in the earth’s mantle. As it migrated to the surface it has remained trapped in underground fields combined with other gasses and in the earth’s atmosphere. The forming rate is too slow to be of any relevance in the timescale of a few human generations. Interestingly, very few studies are being done on the limitations of this resource. Only one research group in the entire world is currently studying the topic. The number of people who know a great deal about the future supply can be counted on one or maybe two hands. One of these is Phil Kornbluth, executive vice president of Matheson Tri-Gas Global Helium. For some background information on Helium I suggest listening to this interview with Kornbluth.
Types of Helium reserves
The sources of Helium on earth can be broadly divided into three categories; 1) Helium rich resources from natural gas fields with a helium concentration of 0.3% or higher; 2) Helium lean resources from natural gas fields with a concentration below 0.3% which mostly is uneconomical to extract; 3) Atmospheric Helium which will likely never be produced because it is too energy intensive to do so. For the two sources of Helium from natural gas, there is little known about the energy costs of production since Helium has so far been produced as a by-product of natural gas production. In this case only produced when the lifetime of the natural gas field and the gas resources warrant the construction of infrastructure to produce Helium for 20 years or longer.
How much Helium is there?
The expected ultimate extractable amounts of Helium are estimated at 40,000 million Sm3 as of 1 January 2007 by the United States Geological Survey (Sm3 = standard cubic meters). Of this amount 93% is endowed in six countries, the United States, Algeria, Canada, China, Qatar and Russia. This ultimate extractable reserve base has been identified using an economic classification, not a geologic one. The reserve classification here includes currently economic, marginally economic and some of the identified currently subeconomic reserves. Of this ultimate extractable amount 7,030 million Sm3 has been classified as reserves that are extractable using existing technology under current economic conditions. However, for Canada, China and Qatar this data is not available which makes the actual figure likely to be much higher. The countries for which figures are available are the United States (3,500 million Sm3), Algeria (1,850 million Sm3) and Russia (1,680 million Sm3).
Since Helium is a by-product of natural gas production, and the discoveries of natural gas fields peaked in the ‘70s of the 20th century and have been on a declining trend ever since, it is unlikely that much more Helium reserves will be discovered. The earth’s endowment of Helium is therefore quite well known although the figures are not very precise. This is due to a lack of a universal methodology to measure Helium reserves. Whether these figure are on the upside or downside is unknown. For reasons of political nature, governments worldwide normally have a tendency to exaggerate reserve figures and as a result it is likely that the expected ultimate amount of Helium in reality is lower.
The consumption & the lifetime of Helium
Figures for worldwide helium consumption are not available since there is no agency that tracks this data. As there only is one storage for helium in the entire world, the Cliffside field in the United States managed by the federal bureau of land management, it can be inferred that world production statistics plus net stock drawdowns or minus net stock intakes in the United States roughly match consumption figures. From these figure it can be concluded that Helium consumption has been rising steadily over time especially since the mid 80’s of the 20th century.
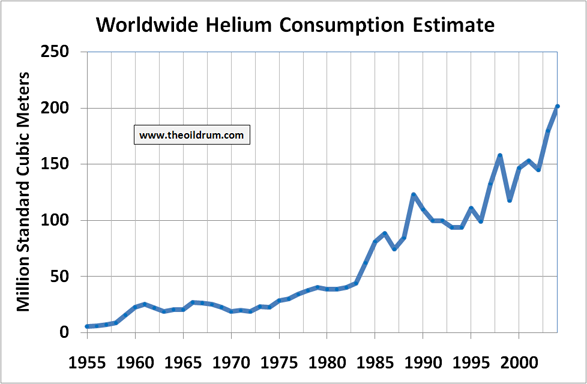
With this methodology a world consumption number of 202 million Sm3 can be derived as of the year 2004. A static approach in which the expected ultimate extractable amounts are divided over present consumption gives a lifetime expectancy of 200 years for Helium. Using a more dynamic approach, in which the average consumption growth from 1990 to 2004 namely 5 percent is continuously extrapolated, gives a resource lifetime of approximately 48 years.
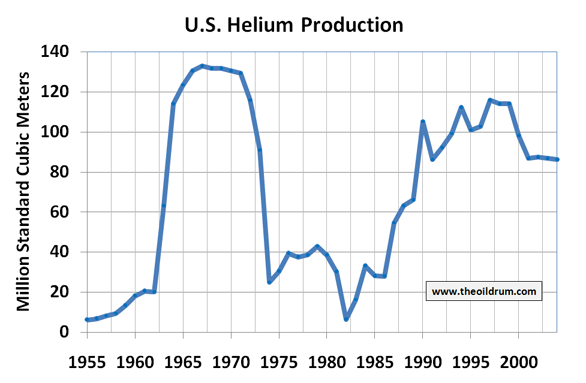
Natural gas peaks, Helium peaks?
However, this does not take into account that the production of a resource never follows a peak and then suddenly drops to near zero. In reality resource production roughly follows a more smooth bell shaped curve due to the physical and economic conditions of extraction. This is also true for natural gas fields and its associated helium.To gain insights into the lifetime of a non-substitutable resource before it runs into its phase of scarcity no matter what technology or investment takes place, it would therefore be better to estimate the maximum possible rate of extraction.For natural gas it has been estimated by independent scientists such as Jean Laherrère that the point of maximum production will be reached somewhere in the period of 2020-2030. Does that imply that Helium will become scarce somewhere in that period? Not necessarily because the number of Helium rich natural gas fields is very small. If some of the natural gas fields are not developed on a speedy basis then the helium production curve will be much smoother and shortages will appear earlier.
Politics and economics come into play
Interestingly, these shortages are already here to a certain extent. The price of Helium rose significantly in the past few years. From a range of 1.5 to 1.8 dollars per Sm3 in the period 1995-2002 to a price of 3 dollars per Sm3 in 2006. The rise in prices occurred due to a scarcity of Helium as a result of production in the United States being in decline since 1999. Being the largest producer of Helium in the world this has led to a gap between supply and demand. This gap is being filled up by stock drawdowns from the United States Helium Reserve. This stock has declined from 985 million Sm3 in 1999 to 696 million Sm3 in 2006.

While sufficient reserves exist to scale up production in other countries these have been slow in response to the decline in production in the United States. This is primarily due to the fact that Helium is a by-product of natural gas fields and operators make a decision not based on the availability of Helium but based on the need to develop Natural Gas Fields. Some new production capacity is coming online in Qatar and Algeria in 2007/2008, but this will not alleviate the situation to sustain a growth rate of 5%. For the near term future therefore, Helium prices will remain high until a large new source of production comes on-stream. The Helium industry recognises this and is forecasting a tight demand-supply balance until at least 2011.
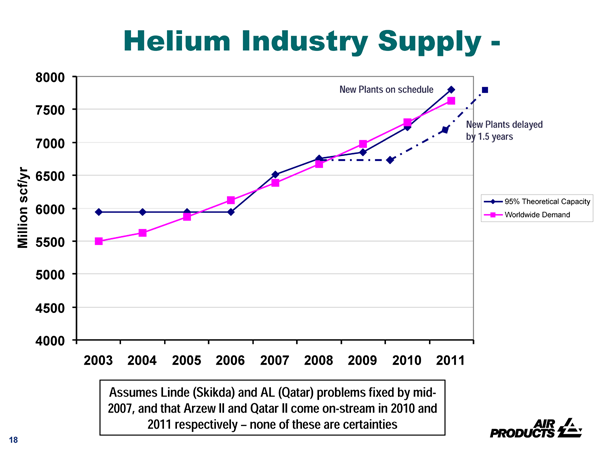
So how long will this tightness last? The first natural gas field that will bring sufficient quantities online that is being spoken about is Kovykta, which lies in Eastern Siberia in Russia. This field contains 40% to 50% of the Russian Helium reserves and is intended to be developed to provide natural gas for China and to provide Helium supplies to the world. Startup is expected to be 2015 at the earliest. So we can expect the present constrained Helium supply to persist until at least 2015, by which the federal helium reserve in the United States will be nearly depleted. If the production of Kovykta is delayed much further beyond 2015 it could lead to severe worldwide Helium shortages.



Its probably worth pointing out that if we were to commercialise molten salt thorium nuclear reactors they produce a steady stream of Xenon. The half life is very short so it becomes non radioactive quickly. This xenon would always need to be removed from the reactor anyway so forms a useful byproduct.
Like helium, its a noble gas. It may be able to stand in for helium in certain industrial operations, but certainly not blimps...
Andy
Andy, there is a little little problem with your Xenon theory. When Xenon 135 emits a beta particle (that is the form its radiation takes.), it also transmutates into Cesium 135 which is not a noble gas.
The main problem is that xenon is no substitute for helium, and vice versa. They're both inert, but in totally different ways.
I was looking at this a few months ago and made these notes:
-- from which the relevant part for this discussion is that fission produces not only xenon-135 but also 136, 134, 132, 131, 130, and 128. These are all stable.
How shall the car gain nuclear cachet?
The number of atoms involved in nuclear reactions is actually pretty small, so the amount of product generated would be miniscle. A chunk of Uranium or Thorium
(or even Polnium 210) is a source of Helium, its just that you don't get very much.
Some high temperature superconductors work at Liguid Nitrogen temps, but generally for any superconductor the colder the better. Losing Helium would be a serious issue. I suspect the price will rise and that should price out the more wasteful applications, and increase recyling in other applications. Still it would be good to increase the market price in anticipation of future shortages.
Ironically Helium is the second most common substance in the universe at large, constituting something like 15-20% of all atoms. But collecting it from the atmosphere of Jupiter would be pretty darned expensive!
Xenon is a very interesting anaesthetic gas with a remarkable ability to protect the brain from hypoxia, it isn't used much because of the expense. Any increase in availability would be a welcome development.
Haven't we been making rather good progress on high-temperature superconductivity recently? Or did I just imagine that?
AFAIK, it's not used in MRI machines yet. They do use cryocoolers instead of LHe in some machines.
For anyone who cares:
Some cryocoolers (specifically pulse-tube cryocoolers) use pressurized helium instead of liquid He. I installed a cryocooler in a lab a couple of years ago to cool a SQUID chip (superconducting-quantum-interference-device) used for magnetic imaging.
Because the He is in gaseous form and in a closed system, the loss rate is much lower than in traditional coolers where the devices used are basically "bathed" in the liquid form. We could still get this chip down to 3-4K with a power output of under 300mW which is pretty impressive.
Yay science!
American Superconductor's 1st gereration superconductor wire operates at around 77K (depending on the local magnetic field, current demsity etc.) and build magnets from this which operate at 20-40K meaning liquid helium cooling is not required. Improvements will no doubt be made on this. I believe HTS magnets will eventually replace rare-earth magnets (such as sintered NdFeB magnets) in wind and wave energy devices, but this is in the distant future.
Richard C
I'm not so sure that you need to wait all that long. There are a number of groups working on this.
Chris
Rembrandt - Thanks for the props in your article. You did a decent job of explaining the helium supply situation. I think that the shortage will be relieved in 2011 by new supplies from Algeria and Qatar, where new plants are expected to be built and one of the existing plants will be expanded.
Also think that Kovykta will happen around the same time frame.
Difficult to forecast beyond three years with accuracy in this business, or most businesses, for that matter.
MrMuileh
@MrMuileh
Kovykta will at the earliest start producing in 2015. I have read some extensive documentation on this matter. As to the Algerian and Qatari supplies, these expansion plans yet have to materialize. Next to that the production declines in the US will continue, making it more wishful thinking that Qatar and Algeria will be able to compensate the situation.
Another interesting application of helium:
We use 'lots' (well, relatively) of helium at our pressure standards lab room. It's used for checking leaks in high-precision pressure gauges and calibrators. The fact that the helium leaks out nicely from even the tinyest of gaps and the fact that its a rare noble gas ie. there is no other source of helium around in the lab so its easy to detect even the tinyest amount - helps reveal leaks and pinpoint exactly their location like no other gas. We could use hydrogen instead but - boom!
Perhaps we could ban the sale of helium those damned celebration balloons to preserve stocks ?
What percentage of helium is used in celebration balloons? I would agree that this is a use that should be eliminated - or perhaps have a very high tax levied, to strongly discourage it.
@Gail
Approximately 7% of world Helium consumption is used for balloon type purposes.
Rembrandt thanks for this article, some good research indeed.
An important article on Helium by Rui Rosa was published a few months ago by SandersResearch, that adds some more pieces to this puzzle:
http://science.reddit.com/info/65rns/comments/
thanks for your support.
I work for a company that manufactures optical filters, and some of our product goes to the space program. In order to properly test our filters designed for space applications we have to cool them to 77 degrees Kelvin using liquid helium. The wavelength passed by an optical filter shifts with temperature changes.
Which brings up the whole issue of testing instruments and structures designed for space - they have to be supercooled for testing. I'm sure NASA uses lots and lots of liquid helium. So it may be: Goodbye helium, goodbye space exploration too!
If you are cooling to 77K you are using liquid nitrogen. The temperature of LHe is about 4K at normal pressure.
Maybe he is using the Helium to flash freeze his veggies as well?
77K is the temp of liquid Nitrogen, of which there is an endless supply at low cost. If that is the temp you need, then you are surely not using helium, or if you are, watch out for that accountant/bean counter walking around.
Cryocoolers can be bought that sit quietly on a desk and make liquid nitrogen from air with a couple of hundred watts of input power off your wall outlet.
Just go to the web and punch in "stirling cryocoolers" and see what I mean.
Sure, those things have helium in them, but not much, and it does not leak out.
NASA does use lots of helium, and not just in the liquid form, although Nitrogen is more ubiquitous. Helium is used to pressurize propulsion system, and to leak test systems, and a host of other uses in the space industry. But fairly recently, the topic of Helium usage actually came up in a meeting that I was in. One of the engines under consideration at the time (about 2 years ago) for the Ares V heavy lift launch vehicle was the RS-68 made by Rocketdyne, and currently used on the Delta IV expendable vehicle. The Constellation program office was considering using 5 of these engines on the first stage of the Ares V. One of the issues that we brought up was the massive amounts of helium required to spin-start the engines during pre-launch operations. It is a very large amount. Let me put it this way...from what I remember, Boeing had to plumb an 8 inch diameter line to the Pad to accommodate the amount of Helium needed to spin-start one RS-68 engine for the Delta IV. The Ares V is going to use 5 of these engines.
I remember the discussions. Things were said such as, "If we spin up and then abort, will there be enough short term Helium availability in the US for a second attempt?"
This issue is attempting to be mitigated (pdf).
Now, that being said, rocket launches don't come along every day....but little kid birthday parties with Helium balloons do ! :)
Helium is a precious resource, but different from a hydrocarbon.
To get the value from a hydrocarbon, you have to burn it up.
Helium never gets used up, unless you disperse it.
And as the world's smallest molecule, it really wants to disperse.
This suggests that the price will go up dramatically and
applications that conserve their helium will not be greatly affected.
The only problem is that, like hydrogen, helium is a really small atom. It's very hard to keep in any type of container, and thus, disperses all on it's own. Like every other resource, its use should be carefully considered and committed to endeavors where something else that might be more easily acquired or reused could be substituted.
As to Gail's question about usage, according to http://www.the-innovation-group.com/ChemProfiles/Helium.htm, "lifting" occupies 15% of helium's usage. I would assume that includes party baloons as well as blimps, weather baloons, etc.
scott
"We must strive to become good ancestors." - Ralph Nader
I feel helium is a critical and finite resource that will never have an adequate substitute for cryogenic applications. We have been very cavalier in using it; I cringe at those huge Thanksgiving Day parade balloons. We ought to be following the Hotelling rule for all that we are worth with helium, and have at least a $50 per cubic meter worldwide tax on the stuff, to encourage sane long term behavior. Once it is gone, it is really gone, and I am sure our great grand children will miss it much for all of the useful things that they would like to do with it. Thanks to us, they will have no choice but to do without.
It is not an absolute requirement that MRI scanners need helium, though many are so designed. They just need an extremely high magnetic field, reliably.
Non-superconducting magnets would work, but they would take more electrical power and generate more waste heat.
There are also less clinically used but scientifically important high quality MEG (magnetoencelography) machines which require liquid helium to get extremely low-noise and sensitive recordings of magnetic fields from human brains under cognition. Here, bathing in liquid helium is apparently the only practical way to get sufficient cooling.
Since the devices are extremely sensitive to magnetic fields, MEG operations preclude any active technologies or metals anywhere in the shielded room. I don't think it would be possible to make any active cooling design which wouldn't overwhelm the sensitive magnetic sensors (which detect individual magnetic flux quanta, smallest possible change in magnetic fields!)
There are other uses in scientific research where liquid helium is essential: very low temperatures needed simultaneously with low electrical/magnetic noise so you can measure very small quantities sensitively.
As well as the supercollider being built in CERN; where the flux intensity combined with physical force and cooling requirements mean that no other solution is feasible for the magnets.
When I was in graduate school, I used prodigious amounts of liquid helium. Even used the gas as well (as a part of the gas mix for a CO2 laser).
For the most part the liquid helium was used to chill superconducting solenoids of one sort or another. Higher temperature superconductors hadn't been discovered yet. Typically we would pre-cool the thing with liquid nitrogen to get the thing down to 77K, blow out the liquid nitrogen and fill with liquid helium. Helium is unique in that it has a low latent heat of evaporation, meaning that it doesn't take much heat to get the stuff to boil off - if you are using it to chill down something that is warmer, it takes a surprising amount of helium to do the job, and it also means that any storage vessels must be exceptionally well insulated (many have a liquid nitrogen jacket to help reduce boiloff).
For some of our experiments we would attach a mechanical pump to lower the pressure over the liquid helium - mainly to lower the temperature below the lambda point (we were doing optical experiments, and the bubbles would screw things up - below the lambda point helium is a superfluid and it no longer boils, and it will never freeze). In our labs, the helium gas on the exhaust of the pump was also vented to the atmosphere, but I suppose it could have been filtered and recycled. If you need the superfluid properties, there is really no substitute for helium. All other cryogenic fluids will freeze if you chill them down enough.
In our labs we had no recycling capability, so the helium gas was vented to the atmosphere. Although some universities did have a helium recycling capability and had helium return lines running through the building. The problem is that along with a recycling capability, you also need a liquifier, and those machines are expensive and consume a lot of power.
We typically used 25 liter storage containers to transport the liquid helium. And of course you were supposed to use a special reaming tool every couple of days and run it down the neck to make sure that an ice block wasn't developing. There was a day where I had completed some experiments, and I had a bunch of such containers in my lab. I was downstairs using the xerox machine, and someone ran in and said that a helium storage container had exploded on our floor. In my mind I saw my whole graduate school career was going down the crapper, but I ran upstairs and found that it wasn't one of mine. Still it was damned impressive how much damage the thing did when it let go. The overpressure in the room blew the doors off the hinges, and the top half of the storage container hit the ceiling and destroyed a lighting fixture. There were pieces of the thing all over the place, and superinsulation all the way up and down the hallway.
In terms of boiling point, the next nearest cryogenic liquid is liquid hydrogen at about 20K IIRC, but the safety considerations tend to nix any ideas of using this for much of anything other than rocket fuel.
I have also used closed cycle chillers. They use helium as a working gas, but since they are entirely closed cycle you just plug it in and turn it on and it chills the thing down that you are trying to get cold. You can't get to temperatures that are quite as low with these things, but for some jobs they work quite nicely. IIRC, you could get to about 10-15K with those designs. As helium becomes more scarce, these things might get to be more common.
So does this potential helium shortage pretty much nix the nuclear industry because of insufficent gas for welding and checking for leaks? Will it also affect the welding of windturbines? Will it even cause shortages of high tech, lightweight aluminum bicycles as helium becomes very expensive? Thxs for any informed replies as this is not my area of expertise.
Bob Shaw in Phx,Az Are Humans Smarter than Yeast?
Inert-gas welding is often done with argon.
Hello GRLCowan,
Thxs for responding. So argon can substitute 100% for the welding and leak-testing %'s in TODer Artax's table in thread above? Or do some metals require helium for welding?
My company does a lot of welding and I am certified in arc welding procedures.
Helium is used mostly in the welding of certain alloy steels and nearly all stainless steel (alloys of chromium and nickel). The welding processes that uses shielding gas are called "metal inert gas welding" (MIG for short) and "tungston inert gas welding" (TIG for short). The MIG process is used for production welding of sheet (especially under 0.125 inch or 3mm), plate and pipe welding. The TIG process is used for smaller production parts and assemblies such as tubing & engine components for aircraft and food handling equipment. The MIG process is more likely used for making high quantity large assemblies such as railroad passenger cars, food processing components and chemical processing equipment. ALL REQUIRE HELIUM FOR WELDING! Use of other gases such as argon will not produce stainless steel welds that are strong and free of porosity.
I would say that passenger transportation vehicles (rail and air) and chemical process equipment are especially dependent on the availability of helium. The conversion of transportation fuel from fossil to biofuel will depend highly on the use of stainless steel and the required helium for welding.
Aufhauser, a maker of welding supplies, makes the following recomendations for stainless welding here:
http://www.brazing.com/techguide/procedures/stainless.asp
Determine appropriate inert Shielding Gas
SMAW - none required
GTAW - Argon is suggested for thicknesses up to approximately 1/2". For thicker sections, argon-helium mixtures or pure helium may be used. Pure helium may also be employed for deeper penetration. The most common tungsten utilized is 2% thoriated.
FCAW - 100% CO2 or Argon/CO2 The voltage may be somewhat lower if argon with 20 to 25 percent CO2 mixtures is selected. Generally, a gas flow rate of 40 cfh is suggested. Adjustments can be made, depending upon the specifics of the application.
GMAW - Spray Transfer Ð Use Argon & 1% to 2% - Oxygen 99% argon, 1% oxygen is predominantly used - 98% argon, 2% oxygen when welding thinner material.
simillar recomendations are to be found lots of other tech sites, this would suggest workarounds for stainless without helium are available by using different procedures. Care to comment?
I have been involved in welding for 28 years. For penetration and weld strength of stainless steel (series 303,304,316 and 321 ASTM material designations) a mixture of Helium, Argon and CO2 is best for GMAW (MIG welding) and GTAW (TIG welding). Argon CO2 mixture can be used but arc temp is less and weld penetration is less along with rougher surface and greater chance of contaminants and porosity. This is especially a problem with pressure vessels and high pressure piping. Much less chance of leakage at weld joints with Helium in shielding gas mix.
Of course thicker stainless steel materials, generally over 0.100 inch or 2.5 mm thickness, can be welded very well with SMAW (arc welding) that requires no added shielding gas as the burning of powdered flux provides the shielding of the weld surface. My point is that many production weld processes need helium for maximum speed of the process and to ensure high quality welds.
argon is used to weld aluminum or any common material that needs a lower temperature to weld, thin steel for example. i dont know about titanium or magnesium.
in the olden days(that was the '60's,i think) aluminum was welded with a heli-arc. it is painful to think about all the helium wasted in those 'olden days. i once asked a welder about using a heli-arc for welding a piece of aluminum and he looked at me like i was from outer space. he is relatively young and had no idea that heli-arc had been used for aluminum.
Most gas shielded Al welding is done using Argon as far as I know. Helium shows up alone or in mixtures when working thick i.e. >1/2" work or with some alloys such as 5 series. Can't speak to nukes, but bikes, windmills, aviation, etc where your talking about alloys like 6061 should not be an issue.
THXS!
Other blanketing gases could be used in many cases. For wind turbines, substitutions of larger parts could be made for a drop in output or perhaps carbon fiber would become the cheaper option because of expensive blanket gas. (Alas, my wind turbine contacts are not good enough to get you details from the overseas engineering staff)
Leak checking in Fission reactors may be a deal-killer for fission.
Upside - excess He if man gets fusion working, right?
Rembrandt:
I think you mean "Magnetic Resonance Image" not "Multi-Resonating-Image" right?
I think you'll find Toshiba and others are now making MRI scanners where the coil is cooled directly by the "cold head / cryocooler" without the need for helium immersion. Improvements in this area compared to conventional immersed coil designs are continuing.
John, Thanks for this remark, sloppy of me. I have changed it in the article.
Fascinating. Wikipedia has an article on helium that says
the major source of helium in the u.s. has been the hugoton field of ks,ok,tx and co. and for a long time was a major source of ng as well. in the '80's when ng prices were deregulated, operators in hugoton petitioned these state conservation commissions to have the well spacing in hugoton reduced so they could realize a higher, market, ng price. another example misguided price controls and of market driven "conservation" too.
If we start doing CO2 sequestration the cost of separating Helium and other noble gas components is going to fall by 99%. There is as little nitrogen in the gas stream as possible so separating CO2/H2O from ArNeKrXeHe in the offgas is not exactly going to be difficult. Ne will be cheap enough to use in ballons though the lift is going to be about one fifth as high. They'll have to use bigger balloons to get a decent amount of lift.
Still it's going to be expensive. The new superconductors don't have very high critical fields, the point at which the magnetic field is as high as it will go and still stay superconducting. That means that the MRI scans won't have as high a resolution using liquid nitrogen cooling. Is there a decent alloy that uses liquid neon to achieve a high critical field point? MgBr or NbSn?
If we start doing CO2 sequestration the cost of separating Helium and other noble gas components is going to fall by 99%.
Afraid not. Neon is around 18 ppm in the atmosphere, helium about 5 ppm (unless you're close to an oil or gas field), krypton about 1 ppm. You've got to process a lot of air to get sizable amounts.
We do process a billion tonnes of air a year to produce 240 million tonnes a year oxygen for steel making. This produces argon that is 1% of air as a by-product. World production of Argon is 0.7 million tonnes per year. At present only a small percentage or air separation plants further extract the rarer gases but they are extracted. 240 tonnes a year of Krypton, which is about five times rarer in the air than helium are produced at about 4.3€/litre. Helium could probably be produced form the air, as it used to be at Linde, at this price or better. Separators on all the worlds air separation plants would yield a thousand tonnes of helium a year.Such a price would preclude trivial uses such as party balloons but would not preclude more important cryogenic applications. Hydrogen can be used as a cryogenic liquid down to 14K which is plenty for superconductivity. NMR scanners have been produced using permanent magnets needing no cooling at all. The decline in the production of cheap helium is a problem but it is not TEOTWAWKI.
@Nick Rouse
Very interesting comments about processing air to produce Helium. I have tried to find literature on this but could not find much. Given my skeptical nature I gave the weak conclusion that helium from the atmosphere might never be produced.
What you are stating is that the costs of extracting helium from the air are somewhat less then 4.3€/litre but in that ballpark, or about a 1000 times more expensive then the present day price of helium. Sounds like a very costly affair to me.
In addition:
"separators on all the worlds air separation plants would yield a thousand tonnes of helium a year"
We are presently consuming around 14000 metric tons of Helium per year. Implying that much more seperating facilities need to be built which will drive up the costs even further.
From a guy who worked 26 years for Linde (now Praxair):
There are a couple of Kr-Xe refineries around the U.S., and several Ne-He refineries. IIRC Chicago has two of each. The refineries are fairly expensive, and you need a big cryogenic oxygen plant to justify them. Usually 1000+ TPD. That size high purity plant was built mainly for the basic steel industry. You don't see many of those built nowadays in the U.S., with the slow death of basic steel here. My WAG would be that the He price would have to quadruple before anyone would even look at smaller plant sizes.
While retrofitting existing large plants is certainly an opportunity, no one will ever build a new plant for rare gases only. Costs too much. You need to be able to sell the oxygen, nitrogen, and argon to justify it.
I'm not an expert on MRI machines, but I've read that the Type II superconductors will not currently do the field strength that the big MRIs require. Neither will permanent magnet machines. Apparently cryocooled or combo LN2/cryocooled are the next big thing.
Assuming that some portions of the world want to pursue high-temp gas-cooled fission reactors: is the amount of helium required for such a reactor significant on the scale of other uses?
@mcain6925
I have no idea, hopefully someone will be able to answer your question
Somewhat... Its just a working fluid however, and while helium is ideal, many other gasses work very well.
It is the only thing that is completely unreactive to neutrons and completely unreactive to all chemical substances.
Isotopically purified heavy nitrogen, (15-N)2, would be a good candidate for poor man's helium, if it didn't cost so much. We have all breathed a tonne or two, I guess, of (14-N)(15-N), but separating out the dinitrogens where both atoms are heavy is difficult.
Billions, trillions, maybe quadrillions of helium atoms will be needed for this.
How shall the car gain nuclear cachet?
CO2 works as well. The neutron capture cross section of most carbon isn't enough to seriously affect the working performance, and I cant imagine you would really go to the problem of doing isotopic separation of nitrogen versus just paying for more helium or using CO2.
Assuming that some portions of the world want to pursue high-temp gas-cooled fission reactors: is the amount of helium required for such a reactor significant on the scale of other uses?
Well, the helium will not go away, will it? It will stay in the atmosphere and can be extracted from the atmosphere, so I can not see any problem here
Olle, in case you haven't noticed yet, Earth is a rocky planet.
Go away? No. Be beyond the reach of man once it passes out of the upper atmosphere and into space? Yea.
I don't see any peak helium problem being in any way similar to the problem of peak oil so I'm not sure what the point of this article is. Helium is used primarily in high technology applications where technology can probably substitute another process, e.g. using high temperature superconductors for MRI scanning. These can already operate at 20-40K and produce fields of up to 25 Tesla. Also, although I have no personal knowledge, I am extremely skeptical that a non-helium based arc welding system could not be developed etc.
The difference with Peak oil is the complete dependence on the current oil based system and the time and investment it will take to move over to a new energy delivery system. I don't see this as being anywhere near the same problem as for helium which is, in comparison, a product for niche applications.
@Crobar
Should TOD write only about energy? That is the issue you raise here. I tend to think that sometimes there can also be room for other interesting subjects regarding resources.
The point of this article is too outline that there is a supply/demand gap in the near term future.
I'm not talking anywhere about long term shortages or that it is not-substitutable. It is not stated anywhere that no new systems will be developed, Although that might be the impression that is given, I agree. Therefore I have added a sentence in the beginning on substitutability.
The point you raise further were already stated at the first sentence of the article, "Some of the great things that make human live much easier are dependent on rare non-renewable resources.".
I accept your point that TOD should expound on other areas and realise I may have been a little harsh in my criticism. My main problem with this article, however, is that non-critical examination of subjects outside the energy sphere may detrimentally effect the site's standing in it's core message.
A newcomer seeing a poorly constructed argument on a top-posted article may be more skeptical of the quality of information on the rest of the site. In my opinion (which of course may be wrong) this particular article was based on a poorly presented premise which does nothing for the credibility of the site. I have no doubt about the accuracy of your supply side arguments, merely on the implications of that supply which are likely less severe than alluded to at the start.
I found the article useful, inasmuch as helium may be representative of a whole class of rare elements with high-tech applications that could be important for a post-peak future (indium in certain types of solar cells may be another). The key point is that helium, like indium, is a by-product, whose supply is not much affected by demand or price, and that the price might have to increase 100X or 1000X for this status to change. When production of the primary resouce commodity (NG for helium) declines or ceases, for whatever reason (including depletion), so presumably will production of the by-product, except at exhorbitant prices.
You raise some interesting points, but I felt the need to point out a few errors. Firstly, MRI is the same thing as NMR imaging - the name was changed because of public perception of the word "nuclear". Secondly, nitrogen freezes at -196C, Helium -269C, not the values you gave.
The other major point is that operating a superconducting magnet at higher temperatures doesn't just mean a poorer scanner - raising the temperature will stop the magnet superconducting and it will quench, meaning it's effectively useless. You might be able to make the magnets out of other materials, but this is (a) expensive, and (b) unless you can get things to superconduct above 40K, you still need liquid Helium to cool them (you can get down to around 40K with other refrigeration techniques at present). It's also not just a matter of getting the magnet superconducting - once you start putting a current through it, the magnet will heat up and the critical temperature actually reduces, so it's not just a case of finding a material that will superconduct above 40K.
And Olle, yes, helium will indeed slowly leach out of the atmosphere into space. It's light enough that there's not really anything to stop it. It's also VERY expensive to extract the little helium that is in the atmosphere and liquify it.
See http://www.amsc.com/products/magnets/HTSvLTS.html for a superconducting magnet which already operates at 20-40K, in addition they state:
"It is also easier to design cryogen-free HTS magnets, an important consideration in underdeveloped countries where the liquid helium mandated for cooling LTS magnets is not available"
It is surely just a matter of time before the technology matures to the point where helium is not required.
@Barneyb
Interesting that MRI is the same as NMR, should have dug into that more deeply. As to the freezing values, for Nitrogen -196C is the boiling point and -210C is the melting point so what is stated is correct. For Helium we are both wrong, the melting point being -272.2C. I have adapted the piece to compensate for this minor error.
Thank you for the insights in superconducting magnets.
Apologies, I misread some of your post - the temps I quoted were both boiling points, which are the temperatures which are usually reached in systems operating with liquid cryogens - it's pretty hard to operate at anything lower than this temperature.
The context in which this temperature is mentioned makes it quite clear that rembrandt is talking about the boiling point, so the value should be -269 °C, as you proposed before. By the way, the melting point for Helium is also incorrect, since it does not freeze down to 0 K. It thus has no melting point.
Shure, some of it goes into the heterosphere from were we also could extract it (I guess), but the only issue I see here is that the price will go up when we have depleted the NG-source, and I can not understand any big problem from that. At least if you compare it with PO and GW
It doesn't just go into the upper atmosphere, it actually escapes into space - Helium is light and fast enough that it can effectively "evaporate" out of the Earth's atmosphere and so would be irretrievable. The small amount of atmospheric helium is constantly replenished through radioactive decay of heavy elements inside the Earth, so it remains at about 5ppm, but purifying from these concentrations would be very expensive.
http://en.wikipedia.org/wiki/Helium#Natural_abundance
Good thing that low-field MRI is starting to mature:
They rely on superconducting quantum interference devices, or SQUIDs, to detect the very low fields.
In cooling, the alternatives to Helium are Neon and Hydrogen. Here is an excerpt from Wikipedia:
Neon is the second-lightest noble gas, glows reddish-orange in a vacuum discharge tube and has over 40 times the refrigerating capacity of liquid helium and three times that of liquid hydrogen (on a per unit volume basis).[5] In most applications it is a less expensive refrigerant than helium.[6]
http://en.wikipedia.org/wiki/Neon ... and for Hydrogen:
http://en.wikipedia.org/wiki/Hydrogen
Helium is only needed for cooling applications below 14K (-259C).
Maybe, maybe not. Helium has some nice heat transfer properties not found in the other rare gases.
Rembrandt,
You have the wrong picture with your story. This is the correct one.
Thanks,
;-)
ziz wrote earlier
"Perhaps we could ban the sale of helium those damned celebration balloons to preserve stocks ?"
Yes, i think this might have to stop, let's just use hydrogen in these party balloons!!!
Just for fun...
i am one of about 20 gas balloon pilots in the USA. Most of us use Helium in our balloons, about one thousand cubic meters per balloon. We fly only once per year, at the big Albuquerque balloon festival. this gas allows us to fly for up to 3 days without landing. good thing we carry a bathroom bucket on board ;-)
Anyway, i thought i would just mention this very unique sport, quite possibly the smallest sport in the world. All the gas pilots in europe use Hydrogen with great success. A few years ago, we paid about $2,500 per balloon fill (1,000 cubic meters) for Helium. We are now paying about $7,500 per balloon IF, IF we can get it. All of the sudden the gas is not available. I realize our use of this gas is irresponsible when we look at all the critical needs, but it seems the quantity we use is rather small compared to the total usage. Also, we are currently migrating to Hydrogen balloons. There is only one Hydrogen balloon manufacturer in the world (In Augsburg Germany), and they are very, very expensive, so it is taking some time to move everyone over. With the dollar a total trash against the Euro, the current price for a new hydro balloon is around $70,000 US. ouch!!!
by the way, the price to fill a Hydrogen balloon in Germany is currently around $300. Yes, three hundred dollars.
Anyway, i just wanted to share some unique information with you guys.
Rembrandt, thanks for a FANTASTIC article! i plan to share it with all the other gas pilots.
best regards,
Phil in Austin, Texas
Phil:
I don't mean to opine as to if you are being "irresponsible" or not, but if you feel that you are then why are you doing it?
Can't you just put hydrogen in the helium balloon? You could add a little ballast to make up for the additional lift. AND no smoking, please!
unfortunately not, the hydrogen balloons are made in a very specific way to make sure no static electricity can occur. Helium balloons are just fabric bags.
Hmm... unlike fossil fuel, helium is abiotic.
I wonder how long it will take the gas fields to replenish themselves from natural alpha sources? (not that we can wait that long, it's just an idle question)
Well, its mostly from uranium and thorium, which have half-lives in the billions of years.
Of course if you're in a hurry you can fission them. The fission products often kick off alphas much faster.
Helium isn't consumed during the reaction, so this isn't much of an issue. It's like saying we'll run out of platinum for our catalytic converters, but in fact we just reuse and recycle platinum from older cars as they are decomissioned. Since it isn't a fuel, we won't run out. As long as it is contained, this isn't much of an issue.
I'm actually more concerned with what we'll do with all the helium by-product produced by millions of years of deuterium-tritium fusion, but that won't be much of an issue for a really long time.