Khosla and I Finally See Eye to Eye
Posted by Robert Rapier on February 20, 2008 - 11:00am
Some people think I am anti-ethanol. That is an oversimplification, and a misrepresentation of my position. I have nothing against ethanol as a fuel. It isn't as good a fuel as butanol, but then again we can't make butanol as efficiently as we make ethanol.
My objection is that I think the way we make ethanol in the U.S. is a big mistake, and we will recognize this eventually. It may happen following a drought in the Midwest that causes corn crops to fail. That may be what it takes before we recognize that recycling natural gas into ethanol via food was a terribly bad and short-sighted idea.
I also dislike the incredible hype associated with cellulosic ethanol. Promising too much lulls the public into thinking we have a solution ready to go in case of an energy crisis. Not so. But underneath that hype is a lot of potential. I don't think cellulosic success will come from an expensive hydrolysis/biological process. This is simply too inefficient, and requires very high fossil fuel inputs. Rather, I think success will come from a thermochemical process.
Lately, I have spent a great deal of time studying this:
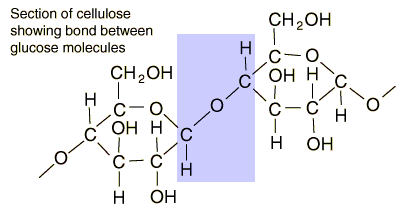
On paper it is deceptively simply to turn that cellulose biopolymer chain into hydrocarbons or alcohols. In practice it is a different matter. If you know your organic chemistry, you can see sites that should be amenable to chemical attack. I have sketched out pathways that seem like they should work, but you never know until you take them into the lab and try them.
One of the things we do in oil refineries is to crack very complex molecules like this. So, for a long time I have wondered about the implications of using various refining processes on cellulose. For instance, can it be cracked in a hydrocracker? How about a catalytic cracker? How would cellulose behave it co-fed into a coker? (There are obvious mass transfer constraints that would have to be addressed).
Imagine my surprise recently when I was trying to determine if anyone has ever done this, and I ran across this:
Khosla Ventures and BIOeCON form KiOR to commercialize cellulosic ethanol
A technology called the “Biomass Catalytic Cracking Process” could be the key to breaking material like wood, grass and corn husks down for ethanol production.
Catalytic cracking is a process already used in today’s petroleum refineries. Simply put, chemicals are used to break down complex organic molecules. The trick is making the reactions between specific chemicals and molecules efficient and controllable, in order to come up with a desirable product like cellulosic ethanol.
The biofuels industry is highly interested in that type of ethanol, but the process of “cracking” the molecular structures of woody plants, whether with chemicals, heat or other methods, has not yet become cost-effective. KiOR is Khosla Ventures’ and BIOeCON’s bet on commercializing a process.
Doh! Looks like I am not the only one who has been thinking hard about this. Clearly I need to stop letting these ideas percolate indefinitely in my head, and write up a business plan and get to work testing them.
I will be the first to admit that Khosla and I haven't always seen eye to eye. But I think his most recent ventures - from Range Fuels to his investments into LS9 to this latest venture - have a much greater chance of success than some of his earlier ethanol investments. Note that none of these processes require an energy intensive, wet-distillation, which has been one of my biggest complaints about ethanol production. I still say that he is overpromising on the potential, but I think he is now heading into more promising waters.
NOTE: "rob@kior" dropped by and commented below that while the press release suggested they are making ethanol, they are actually making a biocrude.



the true cost of ethanol is not measured in dollars or even in eroei. it is measured in declining grain stocks, rising food prices, loss of irreplaceable topsoil and hard to replace fertility. i'm convinced that, in the near future, we'll look back on the bio-fuel frenzy and conclude that it was the product of either political expediency or sheer madness.
Wishful thinking and laziness comes to mind. I have read well thought out critiques of corn ethanol then have the author tout sugar cane ethanol with little or no inquiry into costs.
What do you mean about costs from sugar cane ethanol? Up until recently the majority of ethanol produced was from sugar cane, and most of that without significant subsidies. It seems odd to suggest that cost is an issue that needs a lot of thought when there is a large existing market for the product.
..or to paraphrase the old Hammer saw (can I say that?)..
"To an internal combustion engine, everything looks like potential fuel."
That said, I am always grateful for Robert's work and the thoughts he shares here. He sets a very good precedent for the kind of respectful and researched discussion that will help us find out what can work, what is not working, and why.
I'm not at all against using chemistry, mechanical engineering or other sciences to address these challenges.. just because we've hit our thumbs with that hammer, doesn't mean it can't still drive nails if handled properly.
Best,
Bob Fiske
Robert, What do you think about this?
http://thefraserdomain.typepad.com/energy/2008/02/syntec-cellulos.html
I have always found Syntec interesting, but it wasn't ever clear what they were actually doing. They seemed to be engaged only in catalyst development.
A little bit of hype from the story:
This marks a major milestone for Syntec as this yield is equivalent to revenues in excess of $27 million per year for a 300 ton per day biomass processing facility.
Better than that, it's $27 billion per year for a 300,000 ton per day facility. But they don't have a facility at all, to my knowledge, which makes the $27 million look like a ploy to drive share price.
If you want the real lowdown on them, the poster here who posts as "syntec" works for them. But it still isn't clear what they are doing. It says on their webpage that they have acquired catalyst technology. Maybe Syntec can show up and give us the complete skinny. I quizzed him/her before on this, but I can't recall exactly what the answers were.
The keyword is revenue. Revenue is gross sales proceeds. Revenue does not include cost of goods sold as determined by operating costs, biomass purchases, employee wages, salaries, healthcare, plant and equipment, taxes, interest on debt incurred to build the facility, insurance payments, maintenance, utilities, research and development, etc. There is no gaurantee that there would be any profits at all in such a venture. Many dupes read that tech companies were increasing their revenues in 1999 and 2000, then NASDAQ lost more than half its value and some of these high-revenue companies had to shut their doors and file for bankruptcy as revenue is not enough.
The most profitable use of biofuels other than calories contained in food for people and work animals was the use of cordwood to heat homes. Benjamin Franklin, the inventor of the Franklin stove, said that those who cut and split their own wood were warmed twice. A cord of wood cost $200. A cord of dried wood contained 1.2 tons of wood. If you supply 300 tons of split wood a day you might get revenue of 18 million dollars a year. To operate such a venture you may need a large section of forest, a chainsaw, a truck, a hydraulic log-splitter, a wheel barrow, a helper, some yellow page ads or a sign along a major highway, some bookeeping and tax advice etc. One estimated that a household with a wood burning stove might use 3-4 cords a year.
Don't forget a very large, climate- and pest-controlled wood drying warehouse.
The problem with using wood to heat homes nowadays is that most furnaces are designed for decoration and "atmosphere," not heating. A great number of fireplaces actually cool a house by drawing in outside air.
And that about half the world lives in hot climates. Try burning wood to cool a house (when the outside air is hot too).
Waste much? You're talking about using tens of thousands of watts and greying the sky with air pollution when hundreds of watts (in fans & heatpump) would do.
I'm talking about nothing of the sort. All I said was that it is easier to heat something by burning wood than to cool something. And if you think those of us living in poor hot countriews are the ones who are wasting energy, you are nuts.
A lot of these recent biofuels companies are looking at gasification now(cracking at ~+1000 degrees F).
But how can gasification be more energy efficient than simple distillation(at ~212 degrees F)?
OTH, you can make pretty much whatever you want and it would be relatively carbon neutral and would not use up (as much ) fossil fuel as gasoline. So it's certainly not a terrible idea.
The problem for plain ol' distillation is the enzymes are still to expensive (and fossil fuels still too cheap).
What cellulosic ethanol needs is cheaper enzymes and molecular sieves to separate water from alcohol or cars that can run with more water mixed in the alcohol than IC engines can(ethanol fuel cells).
As I understand things, an internal combustion engine with an Otto cycle can run on 180 proof ethanol. That's 90% ethanol, 10% water. The reason that pure ethanol is needed to make E10 or E85 is that the water in low purity ethanol prevents mixing of the gasoline with the ethanol. There are problems with E85 when water absorbed from the air causes the ethanol to separate from the E85 mix and drop to the bottom of the tank. This might not be a big problem once the fuel is put into a car, but makes shipping and storing the mixture rather difficult and pure ethanol can not be shipped thru a pipeline.
The reason the 15% gasoline is needed in the E85 mixture is to be able to start the engine, especially when doing so under cold conditions. Another solution to this part of the problem would be to use two tanks, one for gasoline and the other for low purity ethanol. Start the engine on gasoline and run that way until warm, then switch to the ethanol tank. With today's computer controlled injection systems and oxygen sensor feedback, this approach should be relatively simple to accomplish. On shutdown, the engine could switch back to gasoline for a brief period to clear the ethanol from the injectors. With cars set to run this way, many small time producers could use local resources to produce low grade 180 to 190 proof fuel ethanol, using heat from local sources, such as wood or solar energy. Of course, the big time oil companies would not like this prospect one bit, as they could not control the marketing and distribution of the ethanol.
E. Swanson
Black_Dog -
Your idea of having a duel fuel system has some precedent with an interesting bit of trivia.
During the 1910s and 1920s mechanical tractors were just starting to make major inroads into US farming practice. At that time, in rural areas kerosene was quite readily available and reasonably inexpensive. Gasoline, however, was not so easy to get and was much more expensive, so some of the early tractors ran a crude Otto cycle engine on kerosene.
But the kerosene first had to be vaporized, and this was accomplished by means of a heat exchanger in contact with the exhaust manifold. These tractors had a single fuel tank separated into two compartments by an internal partition. One side held kerosene and the other gasoline. You started the engine on gasoline and ran it on gasoline until it warmed up and then turned a valve to switch it over to kerosene.
These kerosene-fueled tractors made a distinctive popping sound, and so the farmers nicknamed the early John Deere tractor "Johnny Popper."
I think PHEV cars are the best because you have two fuel options. I think we'll be finding out that having diverse sources of power are a good thing.
The problem with this "solution" (other than the fact that a lot of ethanol is burned) is that it doesn't take advantage of the octane boosting power of ethanol very well. If you're going to keep wet ethanol in a separate tank why not just inject it when you need the octane--according to this:
http://www.autobloggreen.com/2006/10/25/mit-researchers-developing-an-on...
Only about 5% ethanol is required, overall, which, IMO, is the limit we should impose on ourselves in this ridiculous "food-to-fuel" frenzy we are embarking upon. Driving up food costs for those least able to afford it to satisfy and SUV's does strike me as immoral. Feed a Hummer--starve 10 children to death.
But, I digress--a similar trick was used in aircraft during WWII using (smaller amounts of ) pure water when extra power/range was need. (Lugging the engine, gave you the extra range, but also needed a fuel mixture with high anti-knock characteristics).
The popping sound that's heard when kerosene is fed into a gasoline engine is knock or ping--very detrimental to an engine. (next post)
Thanks for the link, HvyOilGuy, but you missed the point entirely. Were running out of oil, remember?
The proposed use of a separate fuel tank was to enable the use of "wet" ethanol during most of the operation of the engine. The octane boost for gasoline, which one would expect from E10, was not the reason. I was suggesting another approach to that presently being used, that is, a mixture of 85% ethanol with 15% gasoline, called E85.
As for water injection, I am well aware of its use as an octane booster and there have been various systems marketed which provide this for cars. The newer ones appear to be quite sophisticated. I bought one years ago, thinking to try it on a high compression (13:1) engine project I built back in the 1980's. The injector system is still sitting in the box in my attic. If I ever get around to fixing my Ford SHO's blown head gasket, I may install the device to the engine, since the Yamaha engine in the SHO was designed for high octane fuel, even though it will run on regular. The knock sensor probably cuts the spark advance to allow operation.
As I recall from my college IC engines course, the use of water injection on WW II aircraft piston engines was intended to allow higher supercharge boost, which gave higher effective compression ratios, thus more power and better efficiency.
E. Swanson
Actually, it's you who is missing the point, IMO. We shouldn't be trying to replace fuels we are consuming that are now (or soon will be) in decline on a Btu per Btu basis, especially with ethanol for which the external costs outweigh the benefits, which seem to accrue to the few. We need to move on to a new transportation paradigm that relies less and less on the private automobile.
That being said, if the "blending value" of ethanol to boost compression ratio and efficiency for the entire fuel system, is sufficienly high, it would justify the production of a certain amount to attain this benefit. I think the article states that it does, but only up to about 5% of the total.
When ethanol is used in excess of this amount, it no longer yields the benefit that the first incremet did--it basically behaves as E85 does now--which hasn't been received all that favorably by the motoring public.
Once you reach the point where more ethanol doesn't provide any special boost, you have reached the practical limit, IMO.
I apologize that the link I gave wasn't particularly informative, since part of the system also involves the use of a supercharger, in addition to the ethanol injection. Maybe this one is a little better, but still just a "news" story.
www.technologyreview.com/Energy/18304/
The other day, Robert brought up another point - oxidizers simply aren't needed on all cars built for the US market after 1994, which adjust their combustion mixture automatically if they encounter different combustability rather than needing it normalized to certain proportions. Ethanol was a great replacement for other environmentally unsound oxidizers, but it's largely an anachronism in 2008.
edit: Remind me to click the links before responding.
Looks interesting. I'd seen it before, but never quite distinguished the technology from others. It looks like it heavily overlaps with hybrid technology, though. Nice for cheapish sportscars and motorcycles for a higher power:weight ratio than hybrids, but electric start:stop driving, wheelhub motor 4WD, and plug-in ability are more attractive in mainstream vehicles IMO.
Sounds similar to the diesel / veggie oil duality. You can run a diesel engine on No. 1 etrodiesel (-40C), No. 2 petrodiesel(-10C), biodiesel(-10 to 10C), or veggie oil(20C to 45C) equally well, but the fuel all gels below a certain temperature(given) and clogs the fuel line. You need to heat it until the fuel temperature is above its cloud point, so everything remains fully dissolved & viscous. So people use No. 1 petrodiesel to start the engine (and use the coolant system to heat the tank), and then switch over. Analogous systems exist for extreme cold weather and fuel additive tanks.
When you distill fermented ethanol out of water, you have to heat the entire mass of water (say 10 times the mass of the water) up to close to the boiling point, plus put the ethanol-water mixture (azeotrope) through a phase change. When you pyrolyze biomass, you're just heating the dried biomass directly - no water to heat, and no phase change to go through.
Bingo. I started to write exactly the same thing until I scanned down and saw your answer.
But how much biomass(wood=6000 BTU per pound) do you need to make a
a pound of ethanol?
For distillation you have to put the 10% ethanol/water azeotrope thru the phase change with a heat of vaporization of about 11000 BTUs per pound of ethanol (~1000 BTU/10%).
A biomass gasifier(75% efficient?), typically produces
a 500 BTU per cubic foot synthesis gas. To make a pound of methanol would take 34 SCF of synthesis gas based on coal gasification(biomass would be worse). So 500 x 34/.75 divide by 6000 would mean that 3.78 pounds of wood make 1 pound of methanol which has 2/3 the heating value of a pound of ethanol. So 5.6 pounds of wood(3.78/.66), 33600 BTUs would make 1 pound of ethanol.
By gasification you get about 54 gallons of ethanol per ton of biomass. By distillation of corn ethanol you get about 150 gallons a ton.
If distillation makes me boil a lot of water, gasification makes you burn/gasify a lot of wood.
Not the same thing. Roughly half of the gasified biomass becomes fuel [1], while the energy used in distillation doesn't add to the caloric value of the product.
[1] At 17.4 GJ/dry ton of biomass and perhaps 78000 BTU/gallon of ethanol, Syntec's figure of 105 gallons/ton is equivalent to ~50% efficiency. This does not include any useful energy yield from the high-temperature gases of the gasifier. It's possible to do far better than the end-to-end efficiency of Syntec ethanol to combustion engine, but Syntec is far better than biological processes which require distillation of relatively weak ethanol solutions.
If I use Syntec and your number above I get an energy loss of 78000 BTU per gallon; 17.4GJ/ton=16.5MMBTU/ton x 50%/105 gal=78,000 BTU per gal.
Shapouri(1995) gives 37000 BTU per gal of thermal input to dry mill distillery plus we also get 18 pounds of DDGS( animal feed) per bushel ( so corn ethanol does make some food!).
http://www.ethanol-gec.org/corn_eth.htm
I made a math mistake 2000 x 2.8/56=100 gallons per ton, not 150.
But Broin has increased the productivity to 3 gpt.
http://tiny.cc/ot7mN
I'm happy that Syntec is getting close to corn ethanol, but just as some think the carbohydrate path to ethanol is limited, I think the thermochemical approach is inherently limited as well. But good luck to Syntec, I'd love to see 113 gpt cellulosic ethanol.
Shapouri(1995) gives 37000 BTU per gal of thermal input to dry mill distillery
That's just for the distillation step. Total energy inputs are almost twice that. They do calculate a BTU credit back for DDGS, but even after that the energy inputs into corn ethanol come out to be around 60,000 BTUs. Not counting DDGS, the energy inputs are actually around 70,000 BTUs.
Let's go back to basics:
To make ethanol, you have to ferment simple carbohydrates - dissolve them in water and let microbes go at them. Some of the energy will be used by the microbes producing heat and other products, but some will end up in ethanol.
Then, you're left with a 10% ethanol, 85% water, 5% other stuff (the "other stuff" scales up the dirtier you make the carbohydrates).
Ethanol really likes water. It really doesn't like to be separated from water - above a certain point(191 proof), it's even impossible to separate it from water via evaporation (it forms something called an azeotrope). Distillation works on the principle that the ethanol has a slightly lower boiling point than the water, and so the ethanol evaporates first (though of course, this is mixed with steam from the just-below-boiling water), and then is condensed. Usually, you have to repeat this several times to get high-purity ethanol. Everyday water is one of the best coolants around, because the hydrogen bonds involved have such a fantastically high specific heat capacity - it takes a lot of energy to boil water. It is this process that is so energy intensive. Keeping things at the required only 80 degrees celsius or so makes secondary energy recovery difficult (and any attempts at that slow down the distillation process, which relies on cold copper to condensate the ethanol out).
Gasification is an entirely different process based on pyrolysis, which is done entirely chemically - it doesn't rely on living things, just hydrocarbon inputs.
Gasification is very Deep Alchemy, but it doesn't require that you separate the result from 10 cubic meters of water (repeated three or four times to achieve high purity, so let's say 40 cubic meters of water) for every cubic meter of fuel you produce. For comparison, a high-level gigawatt nuclear reactor with a once-through fuel cycle (which are currently being blamed by Georgians for the amount of water being taken out of Lake Lanier) only evaporates about a cubic meter of water per second.
Now, most of that energy isn't wasted absolutely (or the ROI would be close to zero rather than close to 1), but every conversion involves losses, and keeping part of the container at 80C while having part of the container cooled takes a lot of energy.
Was a Paper Mill; Going to be a 65 MW Biomass Power Plant. Cool.
http://biopact.com/2008/02/laidlaw-energy-converts-old-paper-mill.html
Another day. Another gimmick. Another 'revolutionary' technology to save the day! Whoopee!
Why has the American farmer (the worlds smartest and best-educated) overlooked this liquid-fuel cornucopia in his own back forty all these years? Why had he not tapped the potential previously when crude was unusually high at $20 or $40 or $60/barrel? Does $100 or $200 crude tell us he is now ready to become 'energy independent?' Was he so shortsighted previously to ignore this cornucopia?
What has woken up this industry? Wondrous new technologies like butanol, algae lipids, or cellulosic ethanol? Previously unheard-of farming techniques? Newly discovered land? I don't think so. The 'Law of Receding Horizons' would suggest that nothing has really changed except desperation, hype, and subsidies.
None of these gimmicks show a positive energy return. None of the studies consider the energy to collect and distribute, transport the crops and fuels, yet ethanol farmers are now grumbling about trucking costs. Those trucking energy costs were left out of the already questionable energy analysis.
It seems Khosla is desperately searching for a new panacea when the last many did not pan out. I see an ecologic and human tragedy turning food into SUV juice for spoiled American consumers? Sad.
The Western Powers will burn anything, go for any process; because those deprived or scammed, directly or indirectly, are in the the third world. That’s taken for granted. Entirely accepted.
The US has no obligation to sell corn abroad or contribute to wheat stocks. So, if 25% of US corn is turned into ethanol (at great dollar cost and hopeless eroei) it is all good. Until one makes the final reckoning, which will show that investment in ‘alternatives’, and massively in Empire, that is military threat, domination, wars - to ensure access, favorable deals, theft, and so on - is not favorable. Money (plus energy, human savvy) thrown after bad.
Treadmill. With the inputs far exceeding the returns.
on edit; added one word = not.
The post yesterday on the likelihood of stopping Global Warming and the one today on new processes of ethanol production both remind me of Matthew Broderick's movie " War Games" during which the Cray Like computer runs frantically through millions of potential nuclear war solutions before finally deciding there is no way to win. We'll keep Frantically looking but the "business as usual" solution is not there. And still, no one can figure out how to make a shrinking economy work. Good luck to us all.
I think you have not been paying attention. There is a big thread running now by Stuart Staniford (4 billion cars) that has just such a solution. All is not lost.
Notice that Treeman said shrinking economy. There is a whole world of difference between that and a steadily growing economy.
Notice that Treeman said shrinking economy. There is a whole world of difference between that and a steadily growing economy.
Notice that Treeman said shrinking economy. There is a whole world of difference between that and a steadily growing economy.
I don't know; the thing that I found most interesting about the Syntec link was the part about lending itself to "small" applications.
I think the most Amazing Statistics I have ever read were the ones concerning the Incredible increases in the Manufacturing of Airplanes, Ships, etc between 1940, and 1944.
There is, absolutely, no doubt in my mind that we could employ a "cookie cutter" technique, and, using a process such as (possibly) Syntec's, kick up four, or five million bpd in a couple of years.
[[Some villagers melted down good iron and steel--tools, even koorknobs--in order to meet the quotas demanded by the state. Even Mao's personal doctor worried about the wisdom of a policy to "destroy knives to produce knives." ]] Tim Harford, 2005
These words came to mind, as I read your proposal. Harford notes that the steel produced in the (excessively localized?) backyard furnace effort mandated by Mao was not usuable. Obviously the warplane and warship industry performed much better, but I wonder how many lives were spent during WW 2 because of mechanical misfunction.
I think if the state is to venture beyond market design its intervention should be experimental and mostly on the reduction-in-use side of the energy economy. Regulations affecting supply should also encourage experimentation. Cookie Cutters are not appropriate tools in a transitional economy.
None of this to say, that I can't think of places where public ownership of the means of energy production and distribution is the optimal property arrangement.
We need novel ways of doing things. As long as the Law of Conservation of Energy and Matter holds sway, novelty will come from combination alone. Public policy should recognize that markets normally process and adapt to information about novel combinations more efficiently than cookie cutting central committees. Public policy should also be to minimize waste.
That aldehyde carbon on the left-hand glucose unit looks susceptible. But those covalent ether bonds are hard to break when surrounded by largish groups. And we know from about a billion years of evolution, and from the dearth of natural breakdown processes (a few fungi, commensal microbes in cow or termite guts) that this is a truly tough polymer.
Some smallish changes in the polymer's arrangement and you have starch, an easy biological polymer. I think the C-O-C bond is more accessible to enzyme attacks in starch.
A question for you Robert: have 3D structure models been made for the natural cellulases? Are there likely to be a lot of undiscovered cellulases or are most of them known? And have the known ones all been sequenced?
This would seem like a good target for some genome tinkering ... your holy grail would be a bug that digests cellulose, produces butanol, and loves to do it all day :)
I am sure they have models of many of the cellulases, but I suspect there are still a lot of them out there. But as you allude to, nothing is really all that efficient, and plants evolved to resist attack.
You mentioned termites above. I have one more post in the queue, and that's exactly what it was about: An experiment I did in grad school with termites.
Hmm, wikipedia has some information. I should have gone there first:
The cellulose unit is that little arrangement of red and green atoms in the lower left corner. There's obviously a lot of work still to be done in that area of biotech.
"your holy grail would be a bug that digests cellulose, produces butanol, and loves to do it all day :)"
If we could only invent a rumen.
The energy cost to duplicate the cow's stomach (to maintain ph, temperature, pressure; to remove and filter the fuel; and then to purify and distill the liquid) would equal or exceed that of ethanol/yeast fermentation system. But then the byproduct is methane and we already do that with no energy gain in municipal waste treatment plants. Why not duplicate the forest floor and bioengineer fungi? Don't they expell CO2?
Well since I was speculating about a maybe possible, but not yet extant, organism, I wouldn't put it in a rumen. As amazing as a rumen is, it probably involves the cow's own immune system as well as various rumen glands (about which I know nothing).
My hypothetical bug would only require a clean environment, to exclude competing / grazing wild bugs, and a supply of nutrients and cellulose. Ideally it would metabolize just enough of the cellulosic sugar to survive (and propagate), converting most of it (via some broken/modified fat synthesis mechanism) to butanol. It would also somehow survive the toxic butanol, ejecting that byproduct in concentrations great enough to simply skim off the vat.
Actually, I think that's Robert's hypothetical bug as well. Since he knows more about it than I do, he'll have a lot of interesting bioengineering problems to solve. :) :) :)
Actually, I think that's Robert's hypothetical bug as well.
It is. My hypothetical bug would consume waste or marginal biomass, and excrete a water insoluble fuel that could just be skimmed off. That's not too far from what LS9 is trying to pull off.
Out of curiosity, does a rumen excrete a simple carbohydrate mix (to be digested farther down the line), or does it process the stuff into the bloodstream itself?
In the debate on the best biofuel option - we should include discussion of pyrolysis / biochar / bio-oil systems as well. The duel problems of climate change and energy production cannot be divorced. Biochar systems don't make quite as much usable energy per acre, but they extract carbon from the atmosphere and rebuild soil - very positive side effects.
http://www.css.cornell.edu/faculty/lehmann/publ/FrontiersEcolEnv%205,%20...
Great link on biochar, Grondeau. Very Interesting.
Paging Vinod Khosla ...
Billionaire Branson regrets mindless biofuel support
http://gristmill.grist.org/story/2008/2/17/205023/451
Paging Vinod Khosla ...
Interestingly, he e-mailed me about this story last night.
There seem to be a good number of unexplored thermochemical pathways for making 'energy products' from nonfood biomass. Let's hope at least one of them will work in a broad setting. For example the major product could be electricity for charging batteries, a gas for compressing into tanks or a crude bunker oil to run stationary engines. I regard a premium liquid fuel such as Choren Sundiesel as superior to alcohol based fuel, yet it seems unlikely to scale up.
Other pathways involve plasma, microwaves, supercritical fluids, aqueous Fischer Tropsch, 'electrocracking' and so on. However we may have already seen the 'winning' 2nd gen biofuel in terms of scalability but were disappointed with the EROEI. Like Jack Nicholson in the movie we have to ask 'what if this is as good as it gets?'
Boof -
One thing that makes me a bit less than bullish about the prospects of a major breakthrough in making liquid fuels from cellulosic material is that over about the last 100 years a considerable amount of research has gone into making all sorts of useful products out of wood and various ag wastes.
Some of the places to look for such are the literature having to do with the pulp and paper industry, agricultural engineering, applied microbiology, environmental engineering, pyrolosis technology, and certain areas of organic chemistry. Probably, 99% of this R&D has never gotten anywhere, but it's all there to be perused if one is so inclined.
Given enough money and enough energy input, you can arrange organic molecules into just about any configuration you want. This might be fine for ultra high-value products like pharmaceuticals, but when you are trying to make fuel on a massive scale, such mundane things as material handling, mass transfer, energy input, residual management, and capital-intensity rear their ugly head and often become formidable obstacles.
We like to think of cellulosic material as 'waste' that just happens to be available for the taking. But once you start attempting to harvest such stuff on a massive scale, then a 'waste' becomes a 'crop', and a crop eventually requires nutrient inputs and other needs for it to be sustainable.
Of course I very well could be wrong, but as I see it, fuel from biomass beyond some relatively modest and highly localized level appears to be a technological and environmental deadend.
Thanks for all your informative posts about ethanol, Robert!
The ethanol industry’s success stems from "the massive transfer of money from the collective pocket of the U.S. taxpayers to the transnational agricultural cartel."
Tad Patzek, http://www.taxpayer.net/energy/ethanolprimer.pdf
The forecast ethanol gross margin according to Iowa State University will average just over 50 cents/gallon from Mar 2008 to Dec 2009.
http://www.card.iastate.edu/research/bio/tools/proj_eth_gm.aspx
The US taxpayer is currently paying, via the government, a 51 cents/gallon subsidy (tax credit) to refiners for meeting the federal Renewable Fuels Standard (RFS) mandate.
http://www.taxpayer.net/energy/ethanolprimer.pdf
If this 51 cent subsidy is removed, the ethanol industry would have almost no gross margin and almost certainly a substantial operating loss. This operating loss becomes even worse if this other government protection is taken into account: the US ethanol industry receives a 54 cent/gallon tariff on imported ethanol.
http://www.taxpayer.net/energy/ethanolprimer.pdf
The picture below is from a recent Archer Daniels Midland (ADM), a member of the agricultural cartel, presentation Feb 19, 2008 http://www.admworld.com/naen/ir/presentation.aspx
This picture might look good to ADM but to me it looks like some large yellow out of control cancer which is engulfing valuable farmland which could be used to grow food instead of fuel. Think that food prices are high now...
When will the yellow corn cancer stop growing?
Microwave resonance to crack the bonds.
http://www.patentstorm.us/patents/6106675-description.html
Unfortunately the C-O bond you need to crack for cellulose doesn't resonate in the microwave area of the spectrum.
It would be at a much shorter wavelength, and by the time you could focus enough UV energy on that particular C-O bond, you've invested so much energy that you might as well just burn the cellulose.
Re: Burning the Cellulose...
Is anyone aware of studies that measure the amount of total net energy (not digestible calories, total energy, burned in a calorimeter) you can extract from an average acre of farmland per annum using only sustainable methods, and any monocrop or polycrop arrangement necessary?
I'm fond of jatropha/biodiesel on arid lands, but if we've got plenty of switchgrass and we can't figure out cellulosic ethanol, we can always burn it, and come out with a high-ROI CO2-neutral alternative to coal.
On the ABC evening news ......
Why You Will Pay More for Bread, Pasta
"Blame it on the price of wheat. Demand for alternative energy has farmers planting less wheat and more corn, the key ingredient of ethanol. According to the USDA, since 1997, the amount of farmland dedicated to planting wheat has dropped from 70.4 million acres to 60.4 million, while corn acreage has risen from 79.5 million to 99.6 million. "
http://abcnews.go.com/WN/story?id=4318523
Just a thought.
Cryogenic attack by halogens (even fluorine) might selectively attack the exposed (and I suspect stressed) O bonds.
Alan
Ouch. A solution that's worse than the problem. ... :)
Yeah, fluorine will attack just about anything. It can oxidize oxygen. And because of its strong affinities, fluorine is the most difficult and energy-costly element to isolate. It prefers to exist as fluorite crystals, CaF2, or fluoroapatite.
The O bond we need to break is neither stressed nor exposed, though of course you can burn anything with enough oxidizer.
BTW, that's the main difference between starch and cellulose. In starch the C-O-C bond is exposed. So you can add H2O to that, cleaving the polymer into a couple of units ending in C-O-H. It's how a pressure cooker can soften the sturdiest beans to an edible consistency.
Cheers Robert and thanks for the fine post. I've enjoyed all your lively discussions on ethanol and have learned quite a bit.
I don't think there's anything like a panacea to the oil problem. Even oil itself took years before it became such an efficient driver of our engines or such a versatile feedstock for our chemicals. Though I don't think it likely we'll ever find a replacement like oil, we might well find a number that can fill the gaps and provide us with the time and opportunity to innovate.
For that reason, I hope we rapidly transition from corn to cost and energy efficient cellulose. It does seem things are moving a bit faster than even a few years ago. I wonder if the sense of urgency is due to more people knowing about Peak Oil than let on.
Best wishes and good luck with your ventures!
Rob
KiOR is commercializinng the biomass catalytic cracking technology ('BCC'). KiOR is a JV by Khosla Ventures and BIOeCON. Our press release has been picked up well, though not always entirely correct. We do not produce ethanol. We produce a bio-crude. Our feedstock is any ligno-cellulosic material, like woodchips, bagasse, corn stover, sorghum etc. We can use the harvest remains of food crops as feedstock and will unlock the remaining energy left unused know. BCC creates a base biocrude that can be further processed in existing refining infra structures.Our technology allows food crops to be used more efficiently (for food AND energy).
Rob, thanks for stopping by and clearing that up. It wasn't at all clear to me how you were producing ethanol like that. I would have expected that you would have gotten a hydrocarbon mixture. What you wrote makes more sense.
I put a note at the end of the story to reflect your comments.
Why muck around with any form of ethanol... Why not just go for methanol... Burn coal and collect the CO2 rathen than sequestrate it, add hydrogen from a perhaps renewable source such as offshore wind and put in them in a big blender and bingo you have methanol.. Well not quite but you know what I mean..
My view is that if methanol is good enough for the Champ Car series then its certainly good enough for the rest of us..
I have thought that you could make methane from biomass and then the next stop is methanol, a simple alcohol. If you want a liquid fuel, that seems like a good one. If you want a gaseous fuel, biomethane seems good. There is not enough biomass to solve all of our energy needs, but if it can solve some of them, that is better than nothing.
The chain you suggest is nonsensical.
Methanol from cellulose doesn't require coal, or offshore wind, or sequestration, or hydrogen.
Whether it's "good enough" to run a performance engine on is irrelevant to whether it has the right composition and sources to scale up to be a major source of energy. One could make a high performance car that runs on refined urine if one wanted to - that wouldn't prove anything in reference to the oil problem.
Robert, congratulations; very interesting post.
With reference with some of the comments:
Interesting to highlight that Richard Branson anounced to regret having invested in corn-ethanol in the US; but believes there is a good potential in sugarcane ethanol in other parts of the world; as Mozambique that he has mentioned and stressed the productiity difference…… not to mention EROEI…
There is one Myth about the development of Flex Fuel Technology in Brazil that might interest some of you. It is said that one of the drivers that lead manufactures to invest in a solution that would allow higher blends of ethanol was the discretionary blending that used to happen in Gas Stations all over Brazil. In every gas station in Brazil (more than 30,000 in total ) there is at least one pump of hydrous ethanol (pure ethanol @ 94.6% strengh), this exist since the early 80’s when Brazil was producing cars that would run only on ethanol. What has happen is that over the 90’s because of the price diference at the pump, hydrous ethanol being much cheaper than gasoline (in some regions in Brazil), drivers with normal gasoline conventional engines were blending directly ethanol at gas stations. They were doing E50, E55, E45 at the pump on normal conventional engines to save money in what was called “Rabo de Galo”. Cars were running normally with this higher blending, but manufactures started to fear that prolonged use of higher blending in normal engines could creat problems, and this was happening in many cases in old vehicles with carburators. To solve this problem they invested in research that lead on the development of Flex Fuel Vehicles…. This is at least I myth that I heard a few times in Brazil… If it is true, I don’t know, FFV is a breakthrough technology but I have seen higher blending or Rabo de Galo many times in my life…. as much as I have seeing people adding whatever different things to the gasoline in order to save money….
Another point that I would like to comment is the statement that pure ethanol cannot be shpped through pipelines. In Brazil, pure ethanol is shipped through pipelines. Hydroscopic characteristics of ethanol make shipment, storage, blending and usage more complex – you have to make sure that there is not water contamination…. but as you can see by the article, published today at F.O. Licht website, there are new projects under development, with a lot of money being invested in new pipelines for ethanol:
Petrobras and partners to build Brazil ethanol pipeline
FO Licht's World Ethanol & Biofuels Report
Thursday February 21 2008
Petrobras has approved the creation of a tripartite company with private partners Mitsui & Co. Ltd. and Carmargo Correa S/A to undertake the conceptual and basic phases for the ethanol pipeline that will be built between Senador Canedo, Goiás, and Paulínia, São Paulo. The project, which will be executed under Petrobras' responsibility, also includes a second section that will integrate the Tietê-Paraná Waterway to the Paulínia Terminal.
The ethanol pipeline is part of the Ethanol Exports Corridor, which starts at the Senador Canedo Terminal, in Goiás, and goes through Uberaba, in Minas Gerais, and Ribeirão Preto, Paulínia and Guararema, in São Paulo. From the Guararema Terminal, the pipeline extends to the São Sebastião Terminal on the Northern Coast of São Paulo, and to the Ilha d'Água Terminal in Rio de Janeiro, via the already existing OSRIO polyduct, which will now be used solely to transport ethanol.
Around its main trunk, between Paulínia and Guararema, the ethanol pipeline will be capable of transporting up to 423.78 MMcf (12 MMcm) of ethanol per year, some 141.27 MMcf (4 MMcm) via the Ilha d'Água Terminal and 282.52 MMcf (8 MMcm) through the São Sebastião Terminal.
Petrobras will place the ethanol pipeline at the market's disposal as a transportation service, allowing for efficient ethanol shipment to the export harbours.
In the early 90s, sugarcane farmers could get more on the sugar market than the ethanol market. This led to a shortage of ethanol at the pumps. That prompted the government to talk with car makers to see if they could do FFVs.
Robert Rapier's post was excellent, but that is expected. But the real congrats this time go to the string of posters who have replied to Rapier's post with a collection of fascinating and thought provoking ideas! I would love to be able to link every high school (even college) chemistry class up with the string of posts we have to this point, it would give them hours of exploration following out the terms, ideas and proposals. Excellent string of replies, this is why TOD still remains so addictive! :-)
RC
Well..
I agree, we should not be using food stock corn.
Corn is one of the worst crops to use and my bowels get all twisted when I hear anyone talk about bio-fuel then corn in the same breath. It exhibits to me right off the bat a dreadful and dangerous blend of intellectual laziness and ignorance that is going to yield results similar to what I would expect to see from my 4yo children if I handed them some guns.
Corn requires a massive at the moment petroleum based chemical infrastructure to secure yields, which in turn is hard on the soil... Healthy soil is arguably our most precious of natural resources.
It has a relatively low yield per acre, its a food stock, and it has a horrid EROI when you are making ethanol from it.. Most solid numbers were talking about .8 to 1 returns!
Now they get all excited to see a whopping 1.1 or .2 to 1!!
It is a stupid choice beyond measure... Sugar cane is even worse as it grows in even less places and requires even more resources in terms of water.
While there is not ONE answer to replace petroleum on the whole.. as it is going to take a total reconstruction of our energy infrastructure with us taking advantage of every aspect of alternative energy.. Wind, Solar, Biofuels, Tidal, and all the others at the local level.
There is only one plant that can meet our niche of bio fuels and at the same time assist us with many of the other chemical stocks our modern world has grown accustomed too in the process. It does not require petroleum chemical stocks to flourish from arctic circle to antarctic circle, Manure is sufficient! it aerates the soil. Its roots penetrate deep (up to 10 feet)... Needs no pesticides, or herbicides at all... It yields 30% more ethanol per ton then corn, yields more ton per acre then any other plant, It has a 3 to 1 EROI (with out adding in the added bonus of not needing petroleum ferts and pesticides). We can get two crops per year where I am in chilly Wisconsin (a substantial corn grower)
And would provide us with the industrial resources for over 50,000 products... from toilet paper to plastics..
That plant is HEMP!
It may not be able to do it all on its own.. but it is far superior to any other plant in every way shape and form.