Storm Watch, 27 June 2010
Posted by methaz on June 27, 2010 - 10:42am
Sunday Afternoon Update
As of 4pm ET, model tracks are continuing to shift north, including the NMC's GFDL and HWRF runs which are highly regarded. Don't be shocked if at 5pm or later tonight NHC moves the landfall location closer to the TX border. Still not a direct threat, but could well trigger some evacuations at the southern end of the OCS fields starting tomorrow. As for the DH situation, every mile north means stronger waves and currents - bad for the cleanup, and more concern for the offshore folks trying to cap the well. Stay tuned . . .
Sunday Morning Update
Well, Alex isn't a done deal yet. Last night one of the main global models, the GFS, showed the ridge of high pressure that is supposed to keep Alex confined in the Bay of Campeche breaking down. This morning, the GFS, Canadian, as well as our in-house MM5 models (which use GFS for boundary conditions) are showing the ridge that is keeping Alex headed in a westerly direction weakening and allowing the storm to curve north - right into the central Gulf as a Cat 2 storm. Other guidance, such as GFDL, HWRF, UK, as well as our in-house WRF runs, let Alex creep north a bit before turning it back towards Mexico. NHC is following the latter guidance, which is still more likely for a variety of reasons, but notes in the latest discussion the potential for the northward turn. Here are the latest (8am ET) track maps. Red line is the 5am NHC track, blue line the GFS low-tracker, purple lines the scary (but lower probability) scenarios.
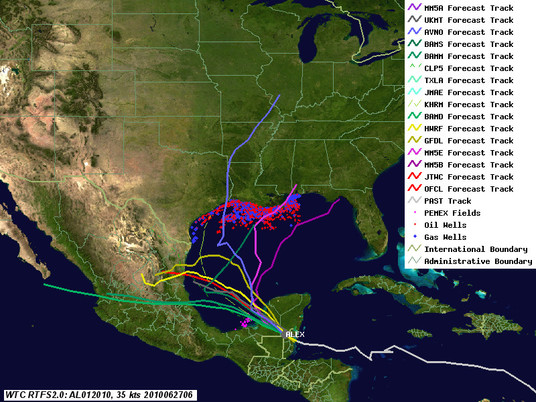
As for the Deepwater Horizon site, forecast wave heights are creeping up as well, as the storm will probably reach a peak intensity of 75-80 knots even if it stays on the southerly course. We're looking at 10 foot waves at least at the Deepwater Horizon site. This bodes ill for the near shore cleanup operations, even if it doesn't impact the operations at the well.
Discoverer Enterprise, the drill ship on-site, can theoretically operate in waves up to 10m (33 feet), but I bet they will bug out before it gets anywhere near that height. Our current bad-case scenario (our MM5E run) is showing 100mph winds and 36 foot waves - and currently, it is #2 in 24 hour forecast track accuracy - 58 miles and decreasing; only HWRF, at 51 miles, is better (NHC is 72 and increasing). My guess is the folks out there are getting nervous. But long term (3-4 days) errors are in the 300-400 mile range for all of the models. Remember that these forecasts, especially for weak storms, are pretty uncertain. It's far from time to panic - these scenarios are just that - possible scenarios! We will have a better picture when Alex emerges tonight.
Original Post
As of 4pm this afternoon, Alex continues to intensify, although it is starting to suffer from some interactions with land, and will probably top out below hurricane strength before it hits the Yucatan and Belize. The big question now is how organized it will be when it re-emerges over the gulf, and how quickly it builds after that. As can be seen from the latest track map, guidance is converging on the central Mexico coast. Below the fold is an analysis of the potential impact of the storm on both the Deepwater Horizon site and current Gulf of Mexico production, along with links to additional resources. Next update will be after the storm exits the Yucatan Peninsula.
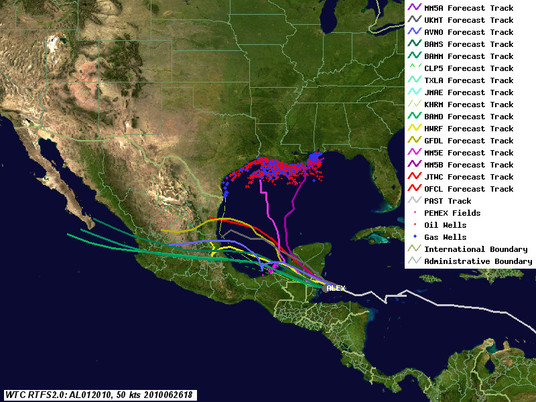
Deepwater Horizon Site Impacts
On these tracks, the biggest risk is from waves in the form of swells. Even these should be moderate, 6ft or less, although one model shows 10 ft. EIther way, that's not too bad for the response ships. However, the waves could be a problem near shore, where breaking waves are tough on protective barriers like booms, and may cause overtopping and currents to transport the oil in to areas currently not oiled. While this isn't as bad as it could be with a stronger storm, it won't help, that's for sure.
US GOM Shut In Production Forecast
As noted in the original Alex discussion, there are many factors that go into forecasting shut in production. For a storm like Alex, the biggest is the decision whether to shut down a platform/rig and evacuate. We probably won't see many evacuations, although facilities off the southern Texas coast may do so. Assuming normal evacuation procedures are followed (which, for a weaker storm, often are not as managers decide to wait it out), we might see a period of 10% shut in for a day or two. No significant damage is expected.
PEMEX impacts
Alex is unlikely to cause much impact on the PEMEX fields, as it should be on the weak side of a storm weakened by transiting over land. I'd be surprised if they shut down anything, but it is possible - the PEMEX decision process remains a bit obscure.
Other energy asset impacts
The current tracks don't have any significant impacts on refineries or electrical generators.
Tracking Resources



Will there be an excess of caution, and the Coast guard invoking a 5-day rule?
Has BP considered product storage or surface trapping during high wind (low gas danger) situatins, or are they just throwing up their hands?
How powerful are the thrusters on the position holding rigs?
If the problem is a steady wind direction, or even a slow changing direction, why not hang a low-windage collection barge on a tensioned cable off the back of a rig, with a motor keeping off the slack, but the wind aligning it?
I've lived a total of ten years at anchor, and the wimpiness and lack of technique in these emergency situations is SO characteristic of government agencies.
The shrimpers must be steaming at the committee nanny approach to this oil disaster.
If a collection barge broke loose, it would still be capturable. The alternative is TOTAL RELEASE?
It's a win-win, but there's a teeny risk. I'd be glad to skipper the collection barge if I had a wetsuit and a few dozen tanks of air to breather and an EPIRB.
It looks from those projected trajectories it has a higher chance of going through Mexico, but if it does veer right like a bowling ball thrown by a left hander, hooking towards Florida, will that shift the oil slick east, heavily lathering Fl beaches? That could be a blessing as it moves the oil away from the Gulf wetlands.
Seems the CCW rotation of the storm may sling it west towards Texas, but I really don't know.
I have a couple of questions regarding the relief well - one technical and one hypothetical/technical:
1. How do you install casing in a well bore that is not straight, and may have blind leads where the drill string was backed out and re-directed?
2. When they succeed in intercepting the first well, what would happen if a similar catastrophic failure occurred and a new exit for the oil was created? Would the flow double, or would it be limited by the size of the original well bore entering the reservoir? (Note: This isn't a stealth "the sky is falling!" question; it's a "what are the applicable fluid dynamics of crude oil/natural gas" question.)
Thanks!
CI - The illustrations can be confusing since they aren’t at true scale. The changing hole angle (the “dog leg”) isn't as sharp as they appear. Typical dog legs are around 3 - 7 degrees per 100’. Steel csg, when it’s 1000’s of feet long, is rather flexible and easily follows the well’s dog legs as long as they aren’t too great.
Though it’s not likely no one can promise a RW won’t blow out. The hands on the relief well have a big advantage: they know they are cutting into a wild flow. If we do understand the cause of the original blow out it didn’t happen as a result of a technical inability to handle the situation: it may sound too simple but it seems they just weren’t paying attention IMHO.
If they can't get the well to "walk" in the required direction they can run a metal wedge (whip stock) in the hole and make the well go in the right direction. This is not a fast process.
I asked this question on an earlier thread, but it was a bit late and I didn't get a reply, so if you'll forgive me, I'll ask it again.
Last time I was working on an oilfield (onshore, southern UK), I was scared to death by the possibility of a release of Hydrogen Sulphide (H2S). It smells like rotten eggs, but in some sort of Darwinian nightmare, it attacks your nasal passages and destroys your ability to smell it. Before you know it you have been exposed to it, and you are permanently asleep (a bit like Nitrogen). I spent half my time on the oilfield walking around and checking that the H2S detectors were still in place and still functional. (I'm an engineering safety inspector and was contracted by the oil company to audit their maintenance procedures; I won't say which one because it's kind of in the news and has lots of lawyers.)
If there's a chemist here, perhaps you could answer my question. What are the chances of H2S being released into the atmosphere of the GOM? I'm genuinely worried about it but seeing as there's been so little mention of it, I presume, but don't know, that even if it managed to get to the surface, it would be so diluted as to present little or no danger to the inhabitants of the GOM. So, is there a threat from H2S?
(PS, hopefully not so off topic as to be disrespectful re. the seriousness of the situation in the GOM, but commiserations to our American brothers in the World Cup.
Wytch Farm, eh? Interesting place. You would never know it's there until you actually come upon the gate.
I don't think Macondo is sour. So I don't think H2S is an issue.
valverx:
Which Farm? Very interesting from an environmental perspective. Have no idea what you mean about Macondo being "sour". I'd be grateful for some extra info.
"sweet crude" is low in H2S, "sour", not so much, and this crude has been described as being "sweet crude".
From my school chemistry, a long time ago..I recall H2S dissolves in water. Not saying it all would, but any bubbling up through so much water would be reduced in quantity, and as there is not thought to be any to start with, I guess that is one less thing to worry about.
H2S is quite soluble in water, especially at 4°C like where it's leaking so it's not likely to make the 5000' trip to see the sun. Compare that to methane and ethane on those charts (what the gas is mostly) and you can see why that bubbles up.
Thank you for your condolences and good luck tomorrow.
In another TOD post, I reference the 1980 Hasbah blowout offshore Saudi Arabia (100,000 barrels). Here is another reference which gives you an idea of what can happen:
[edit -- I don't know about the "heavier than air" part, though, as it is only slightly heavier than oxygen.]
Hydrogen Sulphide, H2S, dangers are well known to offshore workers and vessels servicing them are usually equipped with H2S meters and alarms but others, such as fishing vessels will not. H2S is characterised by a ‘rotten eggs’ smell but the gas itself can disable the sense of smell in higher concentrations.
Says Nomad: “Sad stories exist – the Hasbah-6 blowout in the Persian Gulf back in the early ’80s, where much of the crew abandoned the platform for the sea only to be asphyxiated by H2S, which is heavier than air and settled on the water around them."
JoulesBurn:
Have you any concerns that the dangers of H2S might impact on the clean-up workers, or just as significant, might impact residents of the GOM?
Edit: None block.
As other commenters have noted, Macondo has low sulfur content (is "sweet" as opposed to "sour"), so it is not much of an issue -- and even less given the depth of the release, since it does interact with the water.
Thanks, JoulesBurn; I can now put that particular nightmare to bed (thinking of Fuseli, I suppose).
Molecular wt. of
H2S is 34
O2 is 32
N2 is 28
H2O is 18
CO2 is 44
H2S is heavier than atmosphere but not so much as CO2.
It is definitely heavier that a moist atmosphere (with admixture of H2O)
HTH
Good place to note
methane is 16
ethane is 30
PT -- H2S is rare in the GOM. The only place I've seen it is east of the Miss. River delta - more offshore Mississippi. That part of the Main Pass area is a deep extension of the H2S trends (Cretaceous) in Mississippi.
How do new plume estimates compare with Oxygen availability?
NOAA has just acknowledged that the extensive plumes of oil earlier reported are derived from the DWH spill ( http://www.noaanews.noaa.gov/stories2010/20100623_brooks.html ). Perhaps alarmingly they have adjusted upward the earlier cited concentration from .5 ppm to 1-2 ppm. In view of earlier reports of oxygen depletion (down to 30% normal), I wondered whether sea-water oxygen levels are sufficient to support full microbial oxidation of these higher levels.
Consider then the stoichiometry:
CH2 (hydrocarbon) with a gm-atomic wt of 14 requires
3 O atoms with a total gm-atomic weight of 48 for oxidation to CO2 and H2O
a weight ratio of 3.5
That means we require at least 7 ppm of oxygen in the seawater ( 3.5 x 2 ).
I take some comfort that the oxygen solubility in seawater at 10C is 35 ppm
( http://www.engineeringtoolbox.com/oxygen-solubility-water-d_841.html )
End of scare!
Just because a given amount of oxygen CAN be dissolved in sea water doesn't prove that is IS dissolved therein;there are probably many processes that would work to keep the O2 concentration lower than it's max, but not knowing anything much about oceanography, I can only guess at what they may be.
I do know that the photosynthetic base of the salt water ecology exists only down to a few hundred feet, and that below that everything else aerobic pretty much exists on whatever filters down from above, suggesting that O2 levels must be considerably below the theoritical max for the most part in deep water.
Four primary questions seem to be key to understanding whether dead zones are going to develop;the concentration of the oil, the actual oxygen levels, the tolerance of the local biota to low oxygen levels, and the toxicity of the oil.
The oil concentration will vary from the normal background level of just about zero to some unknown upper limit which might be much higher than 2 ppm in some areas, depending on many factors .
The actual oxygen levels, and water temperatures, may be fairly consistent in deeper waters that are moved into and out of the gulf by major currents.But shallower waters are going to be much warmer , and may have little oxygen as a result of low oxygen solubitily at higher temperatures.Otoh, surface mixing with the atmosphere and photosynthetic organisms doing thier thing will raise O2 levels near the surface to near the max at least part of the time.
Some of the local organisms from the tiniest bacteria to the larger fish may die as a result of O2 levels that do not harm most other organisms living in the oily water;some whole communities such as a coral colonies might die as a direct or indirect result of low O2 levels.
And doubtless many living creatures of many different species will die as a direct result of the toxicity of crude oil.
And of course some nasty positive feedbacks are apt to pop out of such a mix of problems, making the sum of the troubles greater than the individual parts would suggest.
I know just about enough enough to frame the questions-I hope.
Surely we have a few marine biologists or other knowledgeable professionals with us tonight who can take it from here.
"OldFarmerMac" , I agree with your analysis of all the open questions that remain to be answered. Let me at least answer one of them, "What is the real O2 level at the depth of the plume?" Samantha Joye of the UGA Dept. Marine Sciences, who originally reported 30% depletion in the plume, provides a graph of O2 vs depth in the region of the plume:
http://gulfblog.uga.edu/wp-content/uploads/2010/05/station34b_watermark.jpg
On the relative O2 recording, just above and below the plume at 1200 M depth, they show 80-90% of the levels at the surface. Consistent with your own supposition that photosynthesis plays a dominant role in subsurface O2 in the upper ocean, the O2 levels actually decline to 50% at 500 M depth, but then increase at benthic depths. Without doubting the intense activity on certain areas of the benthic mud, I would assume that in the plume stratum .. some 500 M higher .. the O2 availability is geological rather than biological.
Edit: Dr. Joye's blog, with some answers to questions on the composition of the plume is here:
http://gulfblog.uga.edu/
I'm not an authority, but
the oxygen depletion is surely due to oxidation of the oil which is likely mediated by microbial metabolism. So microbes might be metabolizing the stuff but will surely slack off as they deplete the oxygen.
The deep ocean does not mix well, so resupply of oxygen to the region where the is oil will be slow. There is enough about this that a hand waving argument can be made the all will be OK. But this needs more than a hand waving argument, IMHO.
I saw a video of an ROV being used to free a marlin that had gotten trapped in the BOP. I had heard claims that there is not much sea life at the depth of the well head, but this is definitely not true. Lots of misinformation in this. I hope I'm not contributing to it. I doubt very much that marlins consume petroleum. But I wouldn't be surprised to read such a claim on the web.
The video of a marlin getting stuck is at least a year old and apparently somewhere in Australia. Due to a slow modem connection, I have not checked out the video, but a version can be found at http://www.youtube.com/watch?v=D4160D7FAVE
Depth and speed records for Blue Marlin are difficult to pin down, but it is basically a surface fish and having caught a few off Guadeloupe, they go deep when you stick a hook in their mouth, but not that deep. (It is only when you have brought them back to the surface that they start to jump,... walk on there tails... and otherwise behave as they do in the movies)
The next link is to a study that indicates they rarely dive much below 150 meters, (roughly 500 feet,) and never below 200 meters. http://jeb.biologists.org/cgi/reprint/166/1/267.pdf
The true extent of deep water sea-life is one of the big unknowns. A recent NYT article on cold seeps in the GOM detailed thriving colonies close to the the blow-out, in fact, several environmentally sensitive areas restricted the permitted areas open for drilling in the Maconda Prospect. www.nytimes.com/2010/06/22/science/22cool.html
From my own experience diving in shallow water, you find plenty of life close to rock and reef formations while the flat sea-floor usually appears to be pretty sterile. There is no reason to suspect that this should be any different at depth and the fact that in the videos from around the well-head we are frequently seeing fish, (dead/dying?) suggests the existence of what was once a fairly thriving ecology.
Tropical Storm Alex should stay west and away from the oil disaster area. It's moving over Yucatan right now headed for the Bay Of Campeche.
NHC appears to be impressed with Alex's northward turn and continued intensification over land.
Forecast Discussion
http://www.nhc.noaa.gov/text/refresh/MIATCDAT1+shtml/270857.shtml?
Satellite
http://www.ssd.noaa.gov/goes/flt/t1/flash-wv.html
While this isn't as bad as it could be with a stronger storm, it won't help, that's for sure.
replica watches