An Update on US Gasoline Stocks and Blending Components
Posted by nate hagens on December 13, 2006 - 10:02pm
Earlier this week we had a post highlighting the puzzling decline in US gasoline stocks in the face of lower prices. Near the end of the post, an observant oildrum.com reader Matt H2o, pointed out that the EIA has recently changed how they report their gasoline and blending components.

A casual reader of the EIA data would be focused on the headline decline in apples (reformulated gasoline) without knowing there has been an concurrent increase in oranges (blending components). Lest this important point be lost in the recent deluge of (awesome) information on this website, here is a guest post explanation by MattH2O.

Just for a little background - I'm a bit of a cross between a journalist and an analyst. I've been covering energy for a while in one form or another, and am currently focusing on renewables.
Anyway, I picked up on a post written by thegoodshipkship earlier in the week commenting on the fall in US gasoline stocks. Given that prices are lower than they were in the summer, crude stocks are still way above the 5-year average and propane and distillate stocks are within range, I thought something didn't quite sound right. [To see the whole report, click here and scroll to the bottom of the page] So I had a look at the numbers.
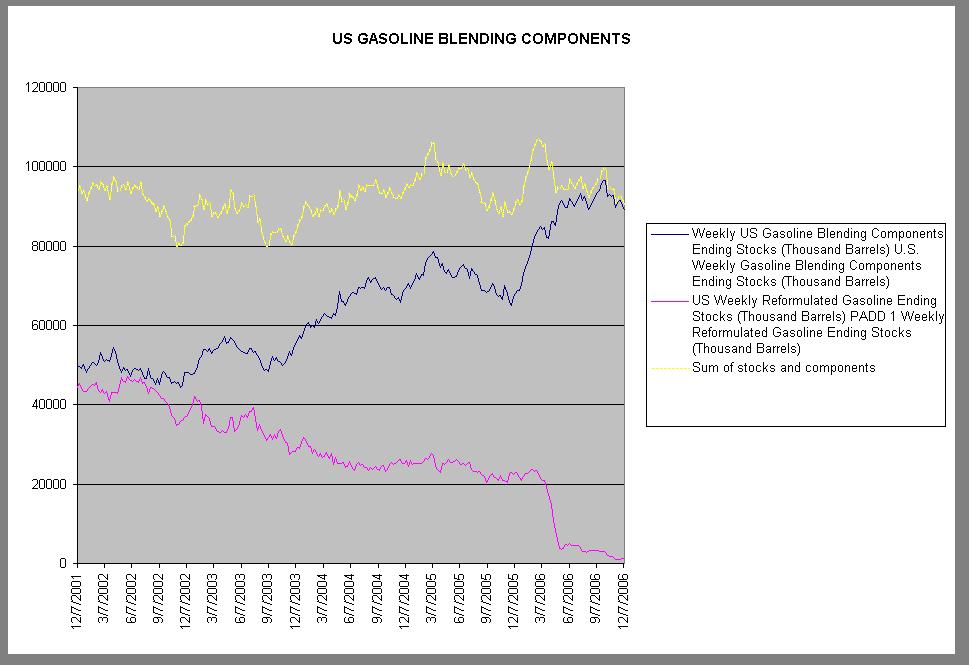
While gasoline stocks are indeed down, gasoline blending components are way, way up. You put the two together and, allowing for the normal Brownian motion, things look pretty level.
There's a very simple explanation for this. The gasoline that's dropping off the chart is finished gasoline - that is, it's ready to put into your car. What has been increasing is stocks of all the parts you need to make finished gasoline. In the aggregate, you have the same total supply of gasoline - it's just that refiners and distributors are storing them separately.
Why the change? Well, this time two years ago, you could store finished gasoline quite happily. But since then, MTBE was phased out and ethanol brought in, partially as a replacement oxygenate, partially because of the Renewable Fuel Standard.
Ethanol has an affinity to water - if you introduce water into a tank full of E10, all the ethanol is attracted to the water and separates out of the fuel mix, leaving you with a layer of RBOB and a layer of ethanol. It's known as 'phase separation'. Irritatingly, you can't just shake it up again like vinaigrette. So refiners and distributors store the two separately, as RBOB (reformulated gasoline for oxygenate blending - basically, RFG sans EtOH) and ethanol.
If you look back at the chart, you'll notice that the big drop in gasoline stocks and the big uptick in EtOH stocks happened at the beginning of 2006, when the RFS kicked in.
In any case, the EIA's charts need updating to reflect new commercial practice...
Anyway, I picked up on a post written by thegoodshipkship earlier in the week commenting on the fall in US gasoline stocks. Given that prices are lower than they were in the summer, crude stocks are still way above the 5-year average and propane and distillate stocks are within range, I thought something didn't quite sound right. [To see the whole report, click here and scroll to the bottom of the page] So I had a look at the numbers.
While gasoline stocks are indeed down, gasoline blending components are way, way up. You put the two together and, allowing for the normal Brownian motion, things look pretty level.
There's a very simple explanation for this. The gasoline that's dropping off the chart is finished gasoline - that is, it's ready to put into your car. What has been increasing is stocks of all the parts you need to make finished gasoline. In the aggregate, you have the same total supply of gasoline - it's just that refiners and distributors are storing them separately.
Why the change? Well, this time two years ago, you could store finished gasoline quite happily. But since then, MTBE was phased out and ethanol brought in, partially as a replacement oxygenate, partially because of the Renewable Fuel Standard.
Ethanol has an affinity to water - if you introduce water into a tank full of E10, all the ethanol is attracted to the water and separates out of the fuel mix, leaving you with a layer of RBOB and a layer of ethanol. It's known as 'phase separation'. Irritatingly, you can't just shake it up again like vinaigrette. So refiners and distributors store the two separately, as RBOB (reformulated gasoline for oxygenate blending - basically, RFG sans EtOH) and ethanol.
If you look back at the chart, you'll notice that the big drop in gasoline stocks and the big uptick in EtOH stocks happened at the beginning of 2006, when the RFS kicked in.
In any case, the EIA's charts need updating to reflect new commercial practice...



From Newsday on 12/08/06:
OYSTER BAY
Crack leads to ethanol spill during transfer
About 3,500 gallons of ethanol spilled into Oyster Bay early yesterday as it was being transferred to a vessel at the Commander Oil facility, Coast Guard and state environmental officials said.
The spill happened at a platform about 100 yards from shore, said Bill Fonda, a regional spokesman for the state Department of Environmental Conservation.
A thread in the piping system connecting the platform with the motor tanker John B. Caddell apparently developed a crack during the ethanol transfer, causing the fuel to spill into the bay, Fonda and Coast Guard Lt. Kris Tsairis said.
Most of the ethanol quickly evaporated, leaving few traces for Coast Guard and DEC teams posted to the cleanup, officials said. "It's almost like rubbing alcohol," Tsairis said. "As soon as it hits the air, it almost starts evaporating. Even our boats, which transited the entire bay, couldn't smell anything. That's a good sign."
The Coast Guard is investigating, Tsairis said.
I had to laugh at this, last time i checked (about a minute ago) EtOH isnt overly volatile, and it is miscible with water. If that ethanol wound up in the bay, it didnt evaporate, it dissolved with the water (its why reason ethanol isnt blended in immediately, as mentioned above). Its good to see the coast guard know all about ethanol. Mind you, it probably dispersed very quickly, though there's a chance some fish got a bit drunk that day.
If I understand the terminology, Ethanol is blended with RBOB to make RFG.
But the idea of using partially burned fuel never made much sense to me, and even the most cursory back-of-the-envelope shows that there's insufficient arable land to replace any significant fraction of our FF use with EtOH anyway. Unless we choose to starve off a lot of people, that is.
That depends on who we are and isn't really a very useful metric anyway. Brazil has replaced over 10% of all liquid fuels and over 25% of all gasoline use with ethanol(both measures on a on a BTU basis).
Thailand, where I live, is about to do the same thing. This to my mind will replace a significant fraction of our oil consumption.
Ethanol from sugar cane has a respectable EROEI and reduces greenhouse gasses from vehicle use by over 80%.
The only known methods to produce energy from solar radiation with a good EROEI are solar cells and thermal solar plants. Everything else is a waste of time, land and taxpayer dollars.
The rainforest was burned primary to feed cattle for meat. There is very little linkage between sugar production and rainforest destruction. Brazil has produced sugar and ethanol for thrity years and yields have improved vastly. They are now working on organic production.
That is just a falsehood. Sugar cane to ethanol has an EROEI of 8-10. I advocate solar, but at the moment ethanol makes more sense from economic and energy perspectives. Engineer Poet wrote a whole poast recently about other ways to transform biomass to energy. Read it.
A reasonable back of the envelope calculation would tell you that for reasonable outputs of cellulosic-based ethanol, the amount of land required to completely replace gasoline usage (on an energy basis) is about twice the land area of all of the US (including Alaska). Since corn is a bit particular about where and how it grows, I can't imagine it growing on the slopes of Denali, Hunter, or Foraker anytime real soon.
If there's enough water in a service station's storage tank to cause phase separation, the ethanol-water phase would sink to the bottom from where, presumably, the retail pump draws its fuel. I suppose the end problem is no worse than it would have been in the bad old MTBE-days, when it would have been pumping straight water. I guess I'll keep driving then.
This analysis seems to fall into the category of searching out every little thing you can conceivably count against ethanol, but not counting positives or charging negatives equally against existing systems.
Corn-based ethanol, especially as applied in the US, is a farce. However, if the US were to import Brazilian ethanol, or ethanol produced in other tropical regions, the 80% reduction in greenhouse gasses over gasoline would certainly compensate for replacing a couple of valves.
Every single car recently built by major manufacturers can be operated with 20% ethanol content with no impact (although the owner's manual probably only says 10%). The retrofits you are talking about would only pertain to a very small portion of the fleet. Engineer Poet has discussed using newly designed engines that inject ethanol separately and further improve performance.
Brazil has had a major ethanol industry for 30 years. Phase separation as such is, as far as I can tell, is never mentioned as an issue that has impacted business.
You may be right on automobiles, but this link talks about expensive and disasterous problems on some boats:
---------------------------------------------------
A serious problem a
CORNYsolutionEthanol has been linked to the weakening of fiberglass gas tanks, clogged fuel filters and carburetors. E10 has a shorter shelf-life than gasoline and also attracts water, causing yet another set of problems. But most alarming is the deterioration of certain gas tanks.
BoatU.S. Magazine reported in January that the ethanol in E10 gas was dissolving a limited number of older fiberglass tanks with potentially disastrous consequences. Independent laboratory tests sponsored by BoatU.S. Marine Insurance have now confirmed that the resins used in some fiberglass tanks are leaching from the tank walls, weakening the tanks.
The resin released by ethanol makes its way through the fuel system where it sticks to valves and other internal engine parts. The buildup of this sticky black substance has bent pushrods, clogged intake valves and ruined some engines. Affected engines may run rough, stall or bog down under load.
--------------------------
The article goes on to list other problems with ethanol in marine engines.
This potentially could be very scary if you and your family experience engine problems while in a shipping lane, or out on the ocean.
Bob Shaw in Phx,Az Are Humans Smarter than Yeast?
I do agree that in a "limited number" of applications in boats, cars motorcycles and other existing (primarily old) equipment, the problems can be severe. I would not advocate mandatory, across the board use of ethanol and do think that people should be made aware of this issue.
I have never claimed that I think ethanol is perfect, can by itself be a major solution to dimishing oil supplies, or will be much more than a temporary band aid.
However, if we think that waning oil resources will threaten our lives, it does not make sense to overlook those applications of ethanol that exist now and are net positive (i.e. sugar cane, not corn or technologies that are not yet developed).
I believe that direct conversion of biomass to electricity will eventually prove to be a much more effective way to utilize plants to offset oil use, but that's not going to help your fiberglass tank owners much either.
Yesterday, a boat with a newish Yamaha F150 was in the shop. The owner said is "quite running".
We were draining the fuel into clear buckets (since the fuel filter did not look good). You could see the clear seperation of the alky and gasoline in the buckets. About 10 percent by volume. He filled up the boat about a month ago from a gas station on the highway. Looks like the fuel absorbed water and seperated after sitting for a month.
I expect to see alot these problems surface.
Obviously a problem in the marine industry.
Main incentives were 1)approx $0.5/g distiller's credit. 2)$0.5/g tariff on imports - recently extended to 2009.
By distributing ethanol nationwide and blending it with "real gasoline" at 10% or less, 1) can be tolerated by gasoline engines, and 2)motorists are obliged to buy it, whether they want to or not - anecdotally, many report about 10% decline in mileage.
Too bad the tools of this policy success couldn't be applied to the more worthy goal of doubling or tripling domestic gasoline fleet mileage.
:-)
Understanding Gasoline Inventories
I expect that the EIA just decided it would be too much hassle to recompile the data, and it might lead to even more confusion if they did so. After all, the difference is fairly marginal (c. 4.5m bbl?) and in a few months the anomaly will have worked its way out of the system.
Just to make clear - I didn't mean that the EIA had changed its reporting. It hasn't - that's the entire point.
Rather, commercial bunkering practice has changed, and the charts that are included in the TWIP report do not reflect the change. The data, as pointed out in that May TWIP linked above, is all there if you look at it properly.
I hadn't seen that May TWIP report, actually - thanks for pointing it out!
matt h2o