Spectral Extravaganza: The Ultimate Light
Posted by Rembrandt on June 13, 2012 - 5:26pm
This is a guest post by Tom Murphy. Tom Murphy is an associate professor of physics at the University of California, San Diego. This post originally appeared on Tom's blog, Do the Math.
 What do you get when you cross an astronomically-inclined physicist with concerns over energy efficiency in lighting? Spectra. Lots and lots of spectra. In this post, we’ll become familiar with spectral characterization of light, see example spectra of a number of household light sources, and I’ll even throw in some mind-blowing photos. In the process, we’ll evaluate just how efficient lighting could possibly be, along the way understanding something about the physiology of light perception and the definition of the increasingly ubiquitous lighting measure called the lumen. Buckle your physics seat-belt and prepare to think like a photon.
What do you get when you cross an astronomically-inclined physicist with concerns over energy efficiency in lighting? Spectra. Lots and lots of spectra. In this post, we’ll become familiar with spectral characterization of light, see example spectra of a number of household light sources, and I’ll even throw in some mind-blowing photos. In the process, we’ll evaluate just how efficient lighting could possibly be, along the way understanding something about the physiology of light perception and the definition of the increasingly ubiquitous lighting measure called the lumen. Buckle your physics seat-belt and prepare to think like a photon.
Spectral Introduction

What do I mean by spectra? Light is characterized by wavelength, running from 400 nanometers (nm, or billionths of a meter) at the blue/violet end to 700 nm at the red end. Some familiar sources emit light at a single, pure wavelength—called monochromatic—like red helium-neon lasers at 633 nm, doubled-YAG (green) lasers at 532 nm, and those very orange low-pressure sodium streetlights at 589 nm (Figure 3). But most natural light sources (or colors) are composed of continuous distributions of light at all visible wavelengths. White light has a healthy helping of all colors, as can be seen in a rainbow, spanning red to violet.
Meanwhile, many synthetic lights (fluorescents; gas discharge tubes such as neon; mercury vapor and sodium vapor lights; etc.) emit a line spectrum—an amalgam of discrete wavelengths associated with atomic and/or molecular energy transitions, possibly with a bit of phosphor thrown in to give some modicum of continuum light.


Colored LEDs (light-emitting diodes, e.g., red, orange, green, blue; Figure 4) are in between the continuum and line sources, emitting light over a continuous—though not very broad—part of the spectrum. Maybe “fat line sources” would be a reasonable characterization.
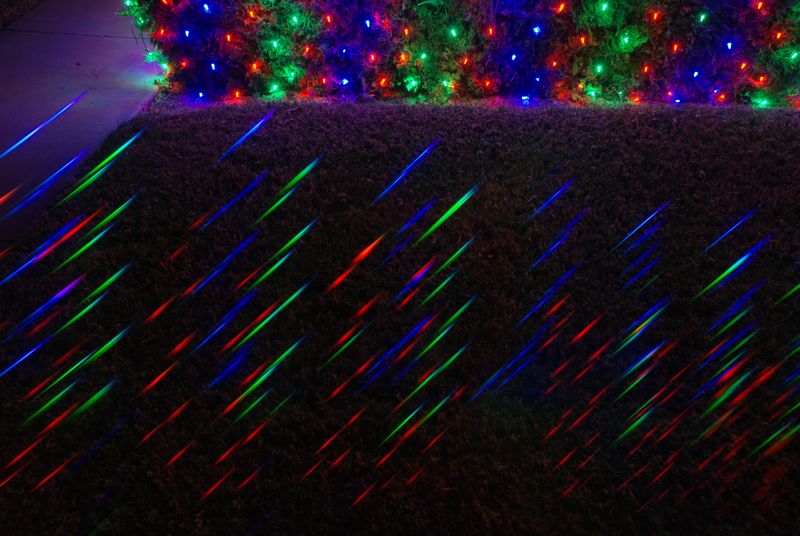
The photos above from around my neighborhood illustrate various forms of spectra in a rich, but sometimes bafflingly complex, way—obtained by placing a transmissive diffraction grating film in front of the lens. The combined-light (un-dispersed) scene is visible in each, but stretching off at an (arbitrarily-set) angle is the “dispersed” version of the same. Line sources produce replications of their shape at each emission wavelength, while continuous sources smear out. The distance from the un-dispersed source to its spectral replica is proportional to the wavelength of light (deep blue/violet is closer, at 400 nm, than deep red, at 700 nm). But the camera helpfully color codes these as well. Note that the low pressure sodium lights are essentially monochromatic—so make a single, sharp, replica at 589 nm. Gas-discharge tubes (as seen in Figures 1 & 2 and intro figure) have a forest of lines. Meanwhile, the colored decorative LED lights produce short, stubby spectra in their “fat monochromatic” way (Figure 4).
Color Perception
Our eyes are essentially tri-color devices. If you look at a sharp spectrum of white light (a rainbow is too mushy, with overlapping colors), you mainly see red, green, and a blue-violet: not much yellow or cyan between the colors. It is only in combination that we create mixture colors. This is why an RGB monitor can synthesize almost any color by varying the brightness of red, green, and blue pixels. Figure 5 shows a representation of the visual spectrum that is not far from our perception, showing the sensitivity curves of the three color receptors in the eye. When we perceive L > M, we call it red, and if we sense L < M, we call it green—until S starts to perk up. I am guessing the S, M, and L labels mean short, medium, and long wavelength.
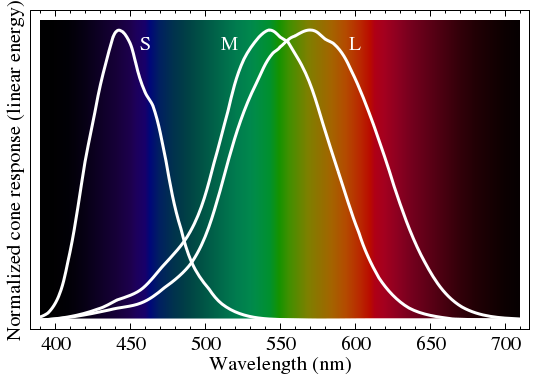
As an aside, if I generate a sharp, continuous solar spectrum for my camera, I see a similar tri-color behavior—albeit much more faithfully red-green-blue (Figure 6). These relate to the filters used in front of the pixels to sort out light by color. Note the absence of very much yellow or cyan in the transitions from red to green and green to blue, respectively.

In aggregate, our visual sensitivity peaks in the green, at 555 nm, falling off on either side in something called the photopic function (there is also a scotopic function for night vision—rods rather than cones—shifted a bit toward the blue). Figure 7 shows the photopic sensitivity function (blue), with a solar-temperature blackbody curve (black) and an incandescent filament blackbody curve (red) also shown for reference.

I will be referring to blackbody spectral distributions, also called Planck functions, after Max Planck. Don’t take the word “black” literally here: think of it as “dull,” as in not shiny like metal. Any “dull” object emits thermal radiation (shiny ones do too, but at suppressed strength). As the temperature climbs, the radiation shifts from the infrared into visible wavelengths, so that a poker may get red-hot; coals get orange-hot, and the Sun is white-hot. These are all blackbodies, and we generally strive to emulate them in our artificial lighting sources. Incandescent bulbs cleverly manage to do this by actually being hot, radiating blackbodies. Their only problem is that most of the light is generated outside of the visible domain. More on this later.
The Lumen
The lumen is a unit that captures how bright a source appears to the human eye. A laser pointer putting out 5 mW of light at 532 nm (green) will emit 3 lumens (lm) of light, while a red laser pointer at 633 nm emitting the same amount of power is only seen to be 0.8 lm. Meanwhile, an infrared laser of any (modest) power output will register zero lumens, since the eye cannot perceive its brightness.
Every photon of light carries a discrete amount of energy, in Joules: E = hc/λ, where h = 6.626×10−34 J·s is Planck’s constant, c ≈ 3×108 m/s is the speed of light, and λ is the wavelength of the photon, expressed in meters (green light is about 5.5×10−7 meters, or 550 nm). So a stream of photons emerging from a light can be associated with a power, measured in Watts, since we can specify how many photons per second are emitted, each carrying known energy. Energy per time is power, and Joules per second is also called Watts.
The lumen is defined so that at the peak of the photopic sensitivity curve (555 nm), one Watt of photon power will carry a brightness of 683 lumens. We can then describe the luminous efficacy of a monochromatic 555 nm source as 683 lumens per Watt (lm/W).
If you stand in front of a selection of light bulbs at the store, you should be able to find the brightness in lumens listed somewhere on the box. You’ll also usually find the actual power consumed by the light bulb. In the case of fluorescent or LED lights, this is not the “replacement wattage” in incandescent terms, but the actual electrical power consumed when the device is on. Dividing these numbers, you’ll find that incandescent lights have luminous efficacies around 15 lm/W. Fluorescent and LED lights tend to be closer to 60–80 lm/W. Much better, but far short of our 683 lm/W monochromatic green source.
Luminous Efficacy of Incandescents
You can figure out the luminous efficacy of any monochromatic (laser) source by gauging how high the photopic curve is at that wavelength relative to the peak, and multiplying this fraction by 683. But what about a distribution? In this case, an integral (piecewise sum of wavelength slices) of the photopic curve times the source spectrum in question does the job.
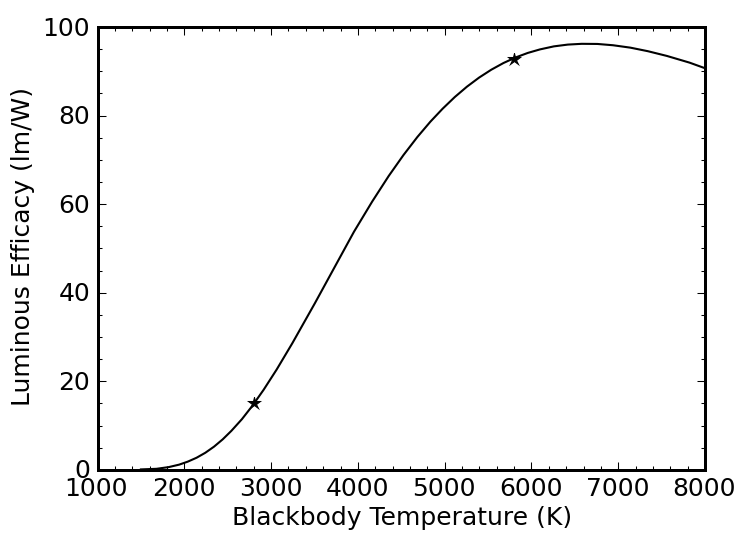
Incandescent lights are essentially blackbody radiators (glowing white-hot), whose spectra are described by the Planck function. Two examples are shown in the plot above, corresponding to the 5800 K surface temperature of the Sun and the 2800 K effective temperature of a light bulb filament. Incandescent lights are phenomenally efficient at converting electricity into photons. The problem is that most of those photons are emitted in the infrared portion of the spectrum, beyond human perception. A 5800 K blackbody emits 37% of its light between 400–700 nm, while only 6% of the light from a 2800 K filament comes out in the visible range.
We can ask what the luminous efficacy of a blackbody source of photons would be. It must be less than 683 lm/W, since many of the Watts are expended at wavelengths of low (or no) photopic sensitivity. The plot below (Figure 8) shows the result. The peak occurs at 6640 K and 96 lm/W, whereas the efficacies at 5800 K and 2800 K are 93 lm/W and 15 lm/W, respectively. Obviously, the solar spectrum achieves near-maximal efficacy. It’s no coincidence: our eyes are adapted to spectrum of our star.
So we could make incandescents more efficient by getting them hotter. The problem is that filaments melt if you get them much hotter than they are already. Halogen bulbs surround the filament with gases that redeposit evaporated atoms back onto the filament, letting them operate at slightly higher temperature. But even these are still far down on the curve.
How Good Can it Get?
Okay, so a blackbody source near 6000 K like the Sun gets up to nearly 100 lm/W. But how good would an ideal white light source be if we could engineer its spectrum to be anything we want? A quick-and-dirty answer can be had by using the fact that 37% of the solar spectrum is within visible limits. If we could design a source to mimic the solar spectrum, but emit not one photon of light at wavelengths we can’t see, we’d get something like 93/0.37 ≈ 250 lm/W.
We can refine this somewhat by recognizing that the eye’s sensitivity at the extreme ends of the visible spectrum (400 nm and 700 nm) is not so hot, so we could truncate our blackbody spectrum more aggressively, and get a higher luminous efficacy by wasting fewer photons where we need them less. The four sets of vertical dotted lines in Figure 7 above represent cutoffs at the 0.5%, 1%, 2%, and 5% levels of the photopic sensitivity curve (blue). In other words, if we were willing to limit the spectrum of our light source to wavelengths at which our eyes have at least 5% of the peak sensitivity, we’d be dealing with the range between the red dotted lines, from about 450–660 nm.
How high will the luminous efficacy climb as we narrow the range? Figure 9 tells the story.
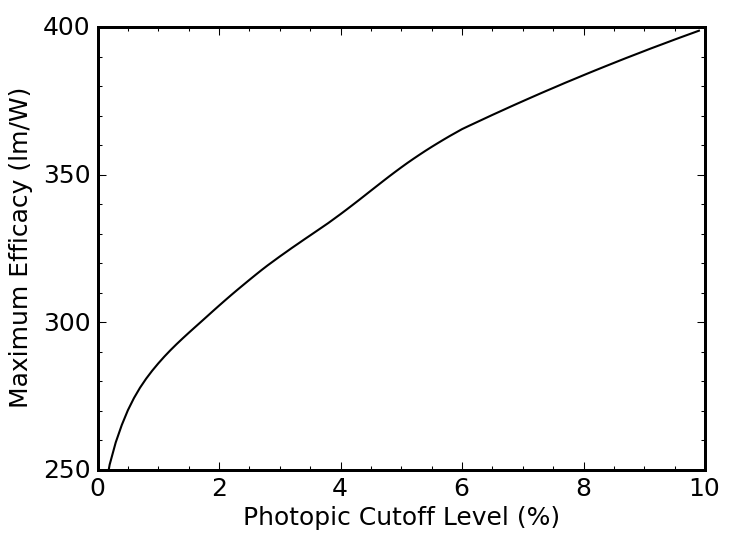
The efficacy gets better and better as we decrease the range. Ultimately, as we approach a cutoff at the 100% sensitivity level, we would be looking at monochromatic light at 555 nm, and a luminous efficacy of 683 lm/W. But this light is useless for color rendering: it’s just green.
So truncating the spectrum comes at a cost. Color rendering will not be as good when we drop the deep reds and deep violet/blues from the spectrum. If we’re going to cut corners for the sake of efficiency, we need to also evaluate the quality of color rendering to establish criteria for an acceptable white light source.
Color Rendering Index
There is a heinously twisted procedure by which one can evaluate the color rendering index (CRI) of a light, given its spectrum. I’ll spare you the details (and secretly wish I had also been spared), but essentially it boils down to “illuminating” eight specially-selected sample color swatches—not particularly attractive colors, I must say—with the test spectrum, and comparing the proximity in color space (think color wheel) to what would happen if the same source were illuminated by a blackbody (incandescent) at the same effective (color) temperature.
Terms that I will be using:
- CRI: Color Rendering Index; ranging from 0–100, with 100 representing perfect blackbody (incandescent) performance.
- CCT: Correlated Color Temperature; the blackbody temperature that results from illuminating a white source. Really, it’s the temperature of the closest blackbody point (called the Planckian locus) in color space. The sun has a CCT of 5800 K, while a typical incandescent bulb is around 2800 K—appearing redder. Higher CCT values look blue, like the light from the stars Sirius or Rigel. This ordering runs counter to intuitive labeling of redder light as “warm” (like fire) and bluer light as “cold” (like ice).
- Planckian Offset: related to CCT, the distance in color space to the nearest blackbody point (on the Planckian locus). Beyond a value of 0.0054, the source is considered to be too far from a blackbody to be considered “white,” and the computed CRI begins to lose meaning.
So if we explore the very best we can do in a truncated solar-type spectrum (CCT = 5800 K)—this time allowing red and blue cutoffs to be at differing photopic sensitivity cutoffs—we arrive at the following plot (Figure 10):

How do we interpret this? Following the solid curve, at the right hand side, we can get a near-perfect CRI around 100 while achieving 260 lm/W. As we increase the level of spectral truncation, the luminous efficacy shoots up (not wasting photons on zones of weak sensitivity), but the CRI declines. Now looking at the dashed line, we find that we exceed the tolerable “white” limit (Planckian offset) of 5.4×10−3 at a CRI of 94 and a luminous efficacy of 310 lm/W. It turns out that this limit corresponds to a wavelength range of 423–659 nm. Thus 310 lm/W is the maximum luminous efficacy for a light we would still regard as “white” at the color temperature of the Sun.
At 2800 K, corresponding to the “warm” illumination to which we are accustomed, we actually do better (Figure 11).

Now, we slide all the way up to about 370 lm/W before busting the Planckian offset threshold, resulting in a CRI of 87 (a little shy of 90, but probably acceptable to most). The wavelength range in this case is 422–648 nm. The red-heavy 2800 K spectrum (see Figure 7) allows a bit more skimping on the red cutoff than was the case for the solar spectrum.
So if we could design a magic light source that emitted a blackbody-like spectrum across a finite wavelength range, generating photons at 100% electrical efficiency (such a source would not even be warm to the touch), we could hope to achieve luminous efficacy values in the range of about 300–370 lm/W. This is roughly a factor of five better than present sources, almost all because of electrical efficiency rather than spectral efficiency.
Example Spectra of Alternative Lights
Enough with the theoretical, magical light. What do real lights achieve? Below is a gallery of example spectra I acquired for various lights. Descriptions follow each one.

Figure 12 shows a 16 W compact fluorescent light (CFL), billed as a “60 W” replacement, rated as producing 900 lumens (so 56 lm/W total). I caught this spectrum just after the light was turned on from a dead-cold state. The spectrum itself has a luminous efficacy of 283 lm/W, at a “warm” color temperature of 2600 K, a Planckian offset well below the 5.4×10−3 limit, and a not-so-stellar CRI of 83.
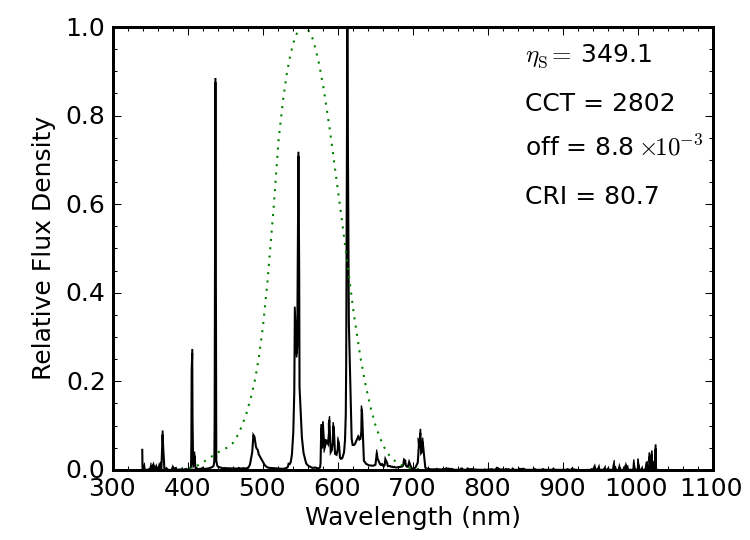
The same bulb after a minute of warm-up has eliminated the forest of infrared lines, and has strengthened its green line relative to the others. The spectral luminous efficacy sails up to 350 lm/W on account of the fact that it no longer produces the infrared lines. But the source busts the Planckian offset limit, and the CRI sinks farther. The light certainly brightened considerably during the warm-up, even though the power draw (as measured via a kill-A-Watt) was steady at 16 W the whole time.
How do we reconcile the fact that a 900 lm source takes 16 W (56 lm/W) when the spectrum is cruising at 350 lm/W? Electrical efficiency. This CFL is turning only about 16% of its electrical energy into non-thermal photons. The photons it does produce are cleverly confined to the visible region (after warm-up), so its spectrum is about as efficient as one might like—even if the color rendering isn’t so hot—but the electrical-to-photon piece is the bottleneck.
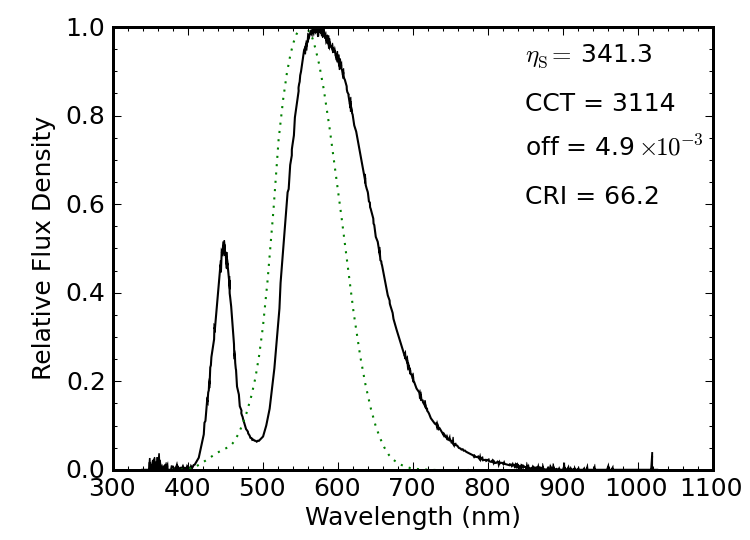
Figure 14 shows the spectrum of an LED light rated at 86 lm and sipping 1.5 W (about 60 lm/W). It’s color temperature is around 3000 K, and therefore “warm.” It’s really just a blue LED (narrow feature at left) pumping a broad yellow phosphor. The spectral luminous efficacy is quite high, at 340 lm/W, and its Planckian offset qualifies the source as being white. Its color rendering is pretty poor, however, at 66. Comparing the spectral luminous efficacy to the achieved luminous efficacy reveals a 17% efficiency at converting electrical energy into visible photons.

A similar LED-based light advertised as having a color temperature of 6500 K is shown above, being just outside the “white” threshold, but having a better CRI (80) while turning in a poorer spectral luminous efficacy of 287 lm/W. The color temperature is measured to be much higher (“cooler”) than advertised. A similar blue LED is used in this light, but with much less of its light being absorbed and reprocessed by the phosphor. The packaging did not present the output in lumens, so I cannot evaluate the electrical efficiency.

A laptop computer screen back-illuminated with a fluorescent source produces a solar-temperature spectrum that is very near the Planckian locus, and with respectable CRI (87) and a luminous efficacy of 317 lm/W. The main three line sources are the same as for the CFL bulb above, but the laptop screen makes more extensive use of phosphors, filling in the gaps between lines a bit more effectively.
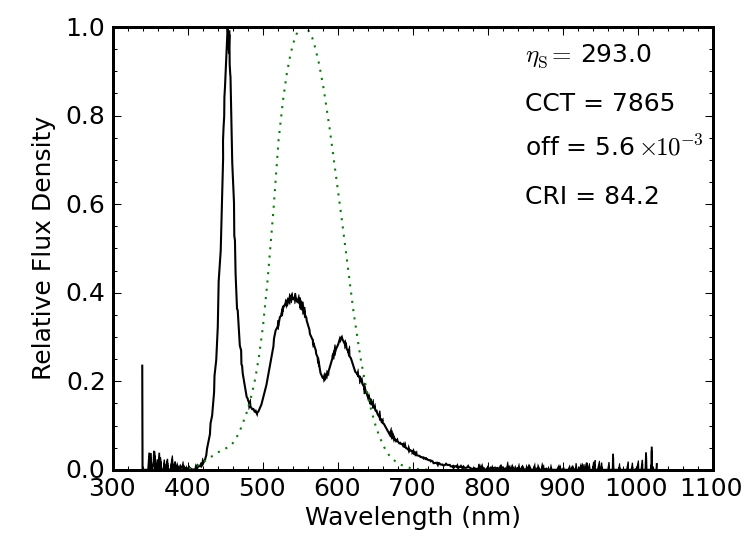
A laptop screen back-lit by LEDs has the familiar blue LED pumping phosphors, but unlike the lights explored above, the laptop LEDs employ an additional phosphor to enhance illumination in the red part of the spectrum. The result is a borderline Planck-like spectrum “cooler” than the solar spectrum, and getting a CRI of 84. The spectral luminous efficacy is 293 lm/W. I also tried an LED-backlit television, with nearly identical results.
Finally, we have a cute device in our physics lecture demonstration facility at UCSD composed of three colored LEDs: one red, one green, and one blue. Three buttons allow any combination to be turned on, the light being thrown into a wedge of lucite (acrylic; plexiglass) with a roughened surface, so the lights diffuse and mix well. Push all three buttons, and you get a reasonable approximation to white. Red plus green (absence of blue) makes the primary subtractive color of yellow. Green plus blue (absence of red) makes cyan. Red plus blue (absence of green) makes magenta (Cyan, Magenta and Yellow—CMY—form the primary subtractive colors for dyes and paints). Sweet little toy. Given these three sources, what is the best mix (varying relative brightnesses) that maximizes the color rendering capability of the three in tandem?
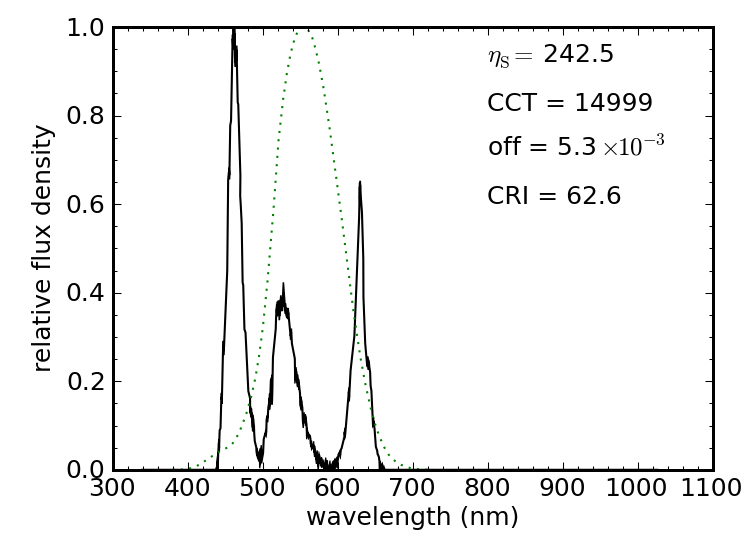
The best I could do is still not terribly good, by lighting standards. If I constrain the source to have an acceptably low Planckian offset, I only get a CRI of 63, and a luminous efficacy of 243 lm/W. If I allow any old offset from “white,” I can get the CRI as high as 77, but the offset is five times the “acceptable” limit (and luminous efficacy hardly budges—up to 246 lm/W). In both cases, the color temperature is absurdly high. The wavelengths of these three LEDs are not at all optimized for this task, so undoubtedly one could do better. Four LEDs (e.g., adding a yellow) would make it even easier.
Lessons
For most readers, this is way more than you ever wanted to know about light sources, spectral distributions, and maximum theoretical efficiency. I warned you at the beginning that this might happen when mixing astronomy and energy efficiency.
Besides being a sneaky way to educate folks about spectra, this post highlights that there is an absolute maximum efficiency achievable by lights. Every photon costs some energy. Even if we had a way of generating visible light photons at 100% efficiency, the photons themselves demand payment. Combined with our physiological sensitivity, and our judgment of suitable color rendering, we find that we will never exceed about 350 lm/W for lights that we would deem to be acceptable.
This means that present efficient lighting (spanning 60–100 lm/W) has only about a factor of four to go before reaching theoretical limits. As with many things, practical realities limit us to some fraction of the theoretical maximum. If we improved lighting efficiency at a rate of 2% per year (35 year doubling time), we would max out within the century.
Meanwhile, as we adjust to progress in lighting, we will find ourselves shaking off the old calibration of lighting intensity tied to Watts. The lumen is the right way to measure perceived brightness. If you keep in mind that standard incandescents are about 15 lm/W, then you can make the calibration yourself. A 1500 lm bulb is at the bright end. A reasonable general-purpose light might be around 600 lm. Flashlights can register in the tens of lumens. A recent excursion to look for LED headlamps demonstrated to me that the lumen is taking off as the primary figure of merit, with numbers typically ranging from 20 lm to 75 lm. I hope to see something like the CRI—and maybe even something akin to the Planckian offset—begin to play a role on the packaging as well, as consumers look for lights that have a natural feel. But who am I kidding? Silly rabbit: the only number people tend to care about is on the price tag.
Aside: The Story of Why
It’s perhaps worth a paragraph to explain why I went to all this trouble to evaluate the maximum theoretical efficiency of white light. It started with curiosity, which—as so often happens—quickly turned into a visit to Wikipedia. A nice table on the Luminous Efficacy page lacked the entry I wanted. So I computed a value for a perfect, truncated blackbody and stuck it in the table. A year or two later, I read a report that quoted a similar maximum, to my delight. I sought the source and found out that it was…me—or Wikipedia, in any case. Looking back at the page, I saw a “citation needed” note. I was surprised to discover that I could not find a published work detailing this maximum. Seeing this hole in the literature, I decided to plug it. A paper conveying much of the content found here is soon to be published in the Journal of Applied Physics. I thought I’d share the juicy parts with those of you having the stomach for this sort of thing. At the very least, it’s a nice collection of spectra of actual household light sources. And even if those bring you no joy, maybe the pictures will?



Thank you Rembrandt, I enjoyed this article.
Thanks, Tom, for another great article. Colour temperature and colour rendering are two critical considerations in my line of work. As I've noted elsewhere on this forum, we can replace a 4-lamp T12 troffer that consumes 160-watts with a new, 2-lamp fixture that draws just 43-watts with no perceivable loss in light output even though my light meter tells me otherwise. We can get away with this because we're typically replacing your standard run-of-the-mill cool white fluorescent (4100K/62 CRI) with one that supplies light at 5000K and a CRI of 82; the higher colour temperature and improved colour rendering of these newer generation lamps tricks us into believing that a space is brighter than it is, and makes for an arguably more pleasant work environment.
The original lighting load at a multi-tenant strip mall that we recently retrofitted averaged between 60 and 65-watts per m2; post retrofit, that fell to just under 16-watts/m2, a near 75 per cent reduction.
In terms of raw lumens, light output was cut by almost half, i.e., 4 x F34T12 lamps x 2,300 mean lumens/lamp x 0.88 ballast factor = 8,096 gross lumens versus 2 x 28-watt F32T8s x 2,675 initial lumens/lamp x 0.77 BF = 4,120 lumens. The gap narrows appreciably once you take into consideration the difference in fixture efficacy -- an estimated 60 per cent for the original prismatic troffers and 85 per cent for their replacements (Lithonia 12-cell volumetric parabolics). Even so, that still leaves us roughly 30 per cent shy of the mark, and yet you would be hard pressed to find anyone who would believe that to be true; quite the opposite. Again, switching to a higher colour temperature lamp accounts for much of the difference.
Cheers,
Paul
Nice data point from Halifax—thanks! Nice when you can get a double-whammy like this and raise no complaints.
I don't know how easy it would be to arrange but a WIP photo, with 1/2 old lights and 1/2 new, on an installation might be interesting and show the advantage, especially a photo like the one above.
NAOM
It's difficult to do a split frame or picture within a picture without losing a lot of detail so I'm afraid I'll have to show these two photographs separately.
Original 4-lamp T12 prismatics
Replacement 2-lamp T8 volumetrics
The three prismatic troffers in the top photograph were fitted with 34-watt cool white super savers and the ones to their right 40-watt deluxe daylight lamps (note the bluish tint). These fixtures consumed 160-watts and 180-watts respectively, whereas the replacement volumetric parabolics draw just 43-watts. Everyone thinks that the store is so much brighter but that's not the case.
Cheers,
Paul
Yeah, that's the problem with 2 photos, it's difficult to give a fair comparison though some of your shots really show the difference. What I was thinking more of, which might work well for the troffers in the above photo, is if you can get a photo after replacing 1/2 of the lights. Another idea might be using a tripod in a well marked spot and aim at a fixed target, eg the A/C vent, then set it up after and use the same target. If you use manual settings on the camera then you should be able to repeat those and get a fair comparison.
NAOM
we could hope to achieve luminous efficacy values in the range of about 300–370 lm/W. This is roughly a factor of five better than present sources, almost all because of electrical efficiency rather than spectral efficiency.
but then it says a somewhat contradictory
This means that present efficient lighting (spanning 60–100 lm/W) has only about a factor of four to go before reaching theoretical limits.
and with lab samples hitting over 200 lm/W and shipping LEDs around 150lm/W, this suggests we are within 2:1 of the calculated ceiling ?
For light that would be acceptably white for most people, yes.
I've been professionally working with battery powered light sources for over a decade now. Power LED's have come from the Luxeon I days of 8 Lumens/watt (1 watt device) to the Cree XM-L at 100 Lumens/watt (10 watt device). I design flashlights that now produce 1000 Lumens from a single LED. I have no doubt that LED's will dominate nearly all human lighting needs in the future.
This article is an excellent summary of what I've discussed over and over with many people at our company and outside of it. It's really so simple! :) So why isn't it perfectly clear to everyone?
Perhaps because they just can't see the light... >;^)
Interesting post. Now I know why some light sources are not very efficient - from a human standpoint. We just can't see much of the light that is emitted!
If the idea of color temps were applied to the high intensity lighting used for streets and highways, a huge amount of power could be saved, IMO. The US has millions of street lights, perhaps as many as 10 to 20 million with each drawing an average of 500 to 1000 watts. If my math is correct, every night this country uses the power of 100 power plants just to keep the roads and other outdoor areas lighted.
Were this lighting of roads and streets be more efficient, say 60% instead of 40%, the power from 30 power plants would not be needed. But then maybe the power cos. like street lighting as it uses extra capacity when demand is down, at night.
Pop quiz. Which would you say is brighter?
This lot illuminated with 400-watt high pressure sodium lamps (460-watts with ballast) at a colour temperature of 2100K and a CRI of 21, generating 45,000 means lumens each.
Or these Philips 330-watt AllStart ceramic metal halide lamps (360-watts with pulse-start ballasts) at 4000K and a 90 CRI, supplying 26,400 mean lumens.
In hindsight, we could have gone with a 205-watt AllStart and bagged an extra 405-watts per pole, or 29.2 kW over seventy-two poles.
Cheers,
Paul
That's an amazing difference in brightness and color clarity. Our company was paying the power company for the cost of having 3 high intensity lights on our warehouse parking lot (rented facility). The cost was over $60 (US) per month on the power bill. Because the power co. would not consider changing to more efficient lamps (they owned the poles and lights), we decided to have them shut off. I am sure the amount of billed electricity could be reduced by half so changing lamps would mean less profit in selling power.
Here is where regulation of power companies could save energy.
The wheels often turn slowly but the transition to LED roadway lighting is well under way here in the Maritimes. Nova Scotia Power is tendering to replace 100,000 HPS street lights in this province and the city of Halifax has been chipping away at its inventory for a couple of years now (there are 40,000 street lights within HRM's municipal boundaries). Our provincial roadways have already been converted (previously a mix of HPS and LPS). Provincial legislation requires that all street lights be replaced within the next five years and so the scramble is on. In neighbouring New Brunswick, NB Power is swapping out 72,000 of their street lights and pole rentals.
BTW, it may not be the fear of lost energy sales that's slowing things down, but rather the high cost of the hardware. The fixtures selected by HRM are $800.00 each and I believe the installed cost is closer to $1,200.00.
Cheers,
Paul
Great example
The poor old sodium lamps look positively jaundiced by comparison (not that they can help it).
Several years ago a colour laboratory which was part of where I used to work, updated their overhead lights from normal fluoros to "daylight" tubes - the same sort of visual impact was immediately apparent with much brighter and warmer light.
Some progress on cheaper LEDs
http://www.technologyreview.com/news/428102/cheaper-led-lightbulbs-are-o...
Thanks Tom, wonderful information.
I really liked seeing the spectrography for the various LEDs vs other lights. Wish I had a tool that could do that. That's not some freeware I-phone App by any chance? (kidding, sort of)
I've just gotten my first little packet of RGB Surface-mount leds, and have wired up one of them on a Three-channel Adjustable Volt Reg circuit, so a bit like the LED toy you use in lectures, I can cross-fade all three to my heart's content to see what mixes are possible. The problem becomes needing a SECOND one, so I have a reference color or just a nice 'harmonic' that will stand in contrast to the first.. otherwise the eye quickly adapts to any lone color and it loses its effect.
I've wondered about adding a yellow, orange, cyan and purple LED and making mixes with more 'tentpoles' to stretch out my Gamut a bit.. but I didn't know which of these is merely a blend of two primary LEDs to start with, adding no new real wavelengths. (Purple, by definition, I would think)
Anybody know?
Thx,
Bob Fiske
Most manufacturers' spec sheets include spectra. Different makers spectra may be different though. Try Cree's site.
NAOM
From Hardhat (below)
"I happened to have a CD with me, so using it as a defraction grating, I reflected the light from the display off it and noticed strong red, green, blue, yellow lines..."
Build a spectroscope from a CD and a cardboard box.
http://www.kidsmakestuff.com/articles/show/53l2
___________________________________________
Adding a white LED allows easy pasteling.
___________________________________________
A nice thing to have is an integrating sphere. Here's one for $4700:
http://www.aviantechnologies.com/products/spheres/standard.php
...or you can use an egg. Empty out the egg shell and admit the lights or mount the LEDs through a hole in the bottom and view the light through a hole high in the side. The integrating sphere will thoroughly mix the colors and present them beautifully in an evenly illuminated window. It is a mistake to paint the exit aperture surround black: the human visual system will bounce, making a ring around the illumination.
___________________________________________
If you put three points down on the color space, select three wavelengths of light sources, you can make any color within the triangle.
http://www.colblindor.com/2007/01/18/cie-1931-color-space/
http://web.canon.jp/imaging/picturestyle/editor/matters04.html
Adding more sources is perfectly legitimate. Many times, the green may not be a pure, deep green like 512nm. Several greens may be something to try because of the curve: 500nm, 512nm, 520nm, 540nm. Yes, deeper blue towards violet or near ultraviolet would add to the gamut. Yes, purple is red and blue... but the single LED colors are single peaks. 560nm is this sickly greenish yellow.
___________________________________________
Yes, the human senses see only difference. If the light, sound, or touch doesn't change, it loses its flavor. The eye is actually in constant motion with saccades. If a mirror is worn on a contact lens so as to hold the image steady on the retina, the planes fade to lines, the lines shrink back to points, and the points wink out. Two light sources could be admitted to the integrating sphere, say top and bottom with viewing through the side, and be switched between. If the switching is at a rate near the scansion frequency of the human visual system, 15Hz, strange things may happen. They certainly do in LED kaleidoscopes with short on times and changing patterns.
http://en.wikipedia.org/wiki/Troxler%27s_fading
http://www.springerlink.com/content/a36v259k3186q683/
___________________________________________
A monochromator is a wonderful thing to play with. It can be a broadband light source, a prism or grating, and an "analyzing aperture" or variable-slit. Turn the frequency knob and the colors flow by. Open the exit slit and the colors go pastel. Close the slit way down and the colors go all sparkly like monochromatic laser light or iridescence.
http://www.youtube.com/watch?v=CwsMYRgtblk
Again thanks, Kaliman.
One of the experiments that has been fun to try with this one, tiny RGB package, is to see how my eye responds to various mixes at very low levels.. The blues and greens seem to hit both rods and cones (could have looked this all up, but where's the fun in that?) .. since just a whisper of B or G light and I can see all the surfaces of the darkened room, while they mostly sink into blackness with redder wavelengths..
And Yes.. wiring my Children's Museum circuits with 'classic' analog chips from the days of disco has got a very compelling beauty and art to it. Wouldn't trade that for anything.. (one part is a series of Jacobs Ladders that count in Binary, with ones and zeros on their opposing faces, in case I hadn't mentioned it several times already.. a very ancient computer!)
The eye is most sensitive to a yellowish green at 555nm.
http://urbangardenmagazine.com/wp-content/uploads/2010/02/human_eye_sens...
Red does not undo night-vision adaptation. Optical telescope users carry red LED flashlights.
The colors of metal surfaces, like silver, copper, or gold, have a picket-fence spectrum with many evenly spaced peaks, say one every 50nm. This is revealed in the study of oil films on water. Look up "surface plasmons".
Several monochromators, each with controls for frequency, bandwidth, and intensity, would make an endlessly revealing color synthesizer.
An error: strike the word "high" - just center the hole in the side of the egg.
Excellent article! While I am sure that LEDs are the future, I am still impressed with the efficiency of even old fashioned fluorescent lights. Some questions:
How many lumens is direct tropical sunlight? What about at 45 degrees north?
Where could I acquire some of this transmissive diffraction grating film? That is neat stuff.
Is there a reasonably priced source of high power RGB single LEDs of decent efficiency? I am interested in making my own lights on a hobby basis such as spot reading lights, wall art illumination, etc.
Thanks again for a very informative article.
Where to find gratings? Google: diffraction grating sheet. Look for something single-axis, somewhere in the neighborhood of 1000 lines per millimeter. The stuff is very cheap!
Defining lumens from the Sun is tricky: the whole sun? Better use lux, or lumens per square meter. Sunlight is about 1000 W/m² times 93 lm/W, or 93,000 lux.
A nice table comparing lux levels can be found at this Wikipedia page.
Thanks for an excellent article. I wish such information was available on the stands of LED salesfolk at trade fairs (having trudged round the London Ecobuild one without learning much - not a single one even seemed to have a luxmeter).
There are folk out there who are very concerned about CRI. Here's a couple of good links:
http://www.alphaled.co.uk/downloads/full_technical_analysis.pdf
http://apps1.eere.energy.gov/buildings/publications/pdfs/ssl/led-color-c...
BobE
funny tried to post last night but got "no comments - closed" message
never mind
This article ties in with the BBC radio program " more or less" I listened to discussing why CFL are not really as bright as you would think. The conclusion was that a political fudge was reach over the comparisons of ordinary candescent light bulbs to CFLS , the correct way to get to the same light levels was to 3x the CFL wattage. Thus the 11W rated "60w" CFL bulb was more or less only 33W candescent.
Again it seems we have a lot of legislation that technology is over taking - LEDs are the way to go by the looks of it.
Forbin
PS: is there any tech that will beat LEDs efficiency ? This one to point out to the cornucopians that we get so far with tech advances - then there's limits to that as well ( usually the 3 laws of thermodynamics ! )
there are some contenders for lighting, but there nothing with the manufacturing scale that LEDs have achieved, it would be very hard to displace LEDs even if something better were available.
One possibility is up conversion phosphors, taking infrared light and up-converting it into the visible region. This is attractive because near IR light can be generated very efficiently (>70% wall plug efficiency) unfortunately the phosphors are still very inefficient.
We considered manufacturing these a couple of years ago but it was clear we could not compete with LEDs
We are working rather hard on LEDs. The trick is band gap engineering. Nature wasn't kind enough to just hand us the proper compounds. But we will get there.
Thanks for the article. Enjoyed it muchly.
I need a little advice...
All my A19 lamps are CFLs, and I am happy enough with them. The room for energy savings in my house is with the various halogen lamps: pot lights, pendants, cable lights and sconces. But I'll have to do it in stages so the first lights that I'd replace the lamps in are the pot lights in the bathroom.
The spec sheet for the housing shows that it uses a magnetic transformer. As well the manufacturer has a selection of LED lamps that will work with the trim (T3450) and housing (flyer).
That said the Phillips MR16 LEDs are readily available through Home Despot (here). Is there any reason why I couldn't use the Phillips MR16 LEDs?
Also Conservation Mart has a whack of MR16 LEDs but I am overwhelmed by the tyranny of choice and unfamiliar brand names.
And the final caveat is that the switch in my bathroom is a vacancy sensor which I assume must be the one meant for Magnetic Low Voltage loads.
Anyway, am I making this more complicated than it is? I guess one off my concerns is with LED longevity in a somewhat enclosed pot light.
It occurs to me that I should probably price the MR16 LEDs that the manufacturer sells and deems compatible first before spending money on the Phillips product.
HiH is really your go to guy here but a couple of thoughts. The transformer may push out a higher voltage at the lower loading of the LED and I wouldn't be sure how that would affect the LED. Your transformers will be lossy, why not think about putting in a modern LED driver and cut the losses there, if you can link the pots then you may be able to drive 2 or 3 together from 1 driver.
NAOM
Philips sells two grades of LED products -- AmbientLED, which is their consumer line sold through normal retail channels and EnduraLED, which are their premium products sold through commercial wholesalers. Whenever possible, go Endura as these products typically offer longer service life, higher CRIs and/or additional light output.
These lamp are compatible with both electronic and magnetic transformers and most leading-edge dimmers. However, they employ an internal cooling fan and the faint, high-pitched whine that they produce drives me nuts. I can't use them in our home for this reason, and would recommend that you try one out before making a major purchase.
Check the packaging to be 100 per cent sure, but I think you'll find that most Philips LED PAR and reflector lamps are not approved for use inside IC (insulated) cans. That hasn't stopped me from doing so, but I accept that their life expectancy could very well be shortened by this.
I have close to two hundred LED lamps in my home, all made by Philips, and with the exception of the aforementioned MR16, I couldn't be happier with their performance.
Cheers,
Paul
"they employ an internal cooling fan"
Where is there room for a cooling fan in this MR16 LED?
It's kinda hard to imagine how this would be possible, but this youtube video shows the internal cooling fan in action: http://www.youtube.com/watch?v=5avJVvrFedc
Related: http://www.youtube.com/watch?v=ktHcMxOfm6g
Cheers,
Paul
Thanks Paul and NAOM for the feedback!
I suppose, given how long they last a little loss of longevity may never be noticed.
I would normally consider switching to using LED drivers now that you mention it NAOM but am trying not to spend too much money or time on this little project. My wife may think I am silly for spending $35 on a light bulb.
Cheers,
Andrew
A sideways thought on this, to give people some different ideas. A computer ATX power supply should be able to drive quite a few 12V LED lights.
NAOM
Our grandchildren will think we were all silly for burning rocks to light up our lives. One 60 watt bulb running one year consumes a BOE ($100?). A $1 bulb is not a bargain.
I've swapped out ceiling can lights and in some cases downsized to the lower wattage LEDs that are selling now at ~$15-$20.
You might have to help your wife a bit with the math. Your grandkids can help perhaps with the moral dilemma.
As ever, I'll toss in my one bit (that being the low-rent alternative to others' two-bits..)
I've now switched out several desk lamps and bedside lamps with LEDs, using 12v Halogen fixtures that I can frequently find at Goodwill or other Thrift sources for around $2-$5.. with the little 20 to 50 watt burned out Halogen Bulb sitting in there behind one little phillips (different Phillips, culturally anyhow, than the quality source that HiH refers us to..) screw, and therefore too much trouble to bother with for most.. and I pop in one of these,
http://www.superbrightleds.com/moreinfo/led-bi-pin/10hp-led-disc-type-g4... for $15
*(this is the side-pin version, there is also a rear-pin version, depending on the orientation of the halogen being replaced..)
..that draws instead around 2.5 watts, well down into the noise level on a Kill-a-watt meter's readings, and end up with this..
http://i831.photobucket.com/albums/zz240/Ingto83/IMGP3069.jpg
http://i831.photobucket.com/albums/zz240/Ingto83/LEDBedstandLamps3073.jpg
..which is still too bright for bedtime 'mood lighting'.. but it's fine for reading!
Thank you for a very informative post.
I was walking past a mall jewelery store a couple of years ago, and the glittering of their product in the showcase about blew me away.. so colorful and twinkly. I couldn't imagine how jewelery could sparkle so. I happened to have a CD with me, so using it as a refraction grating, I reflected the light from the display off it and noticed strong red, green, blue, yellow lines - not a continuous spectra at all.
That prompted me to go into the store for a chat with the clerk, who got the manager, and lucky for me the owner was there. Turns out he had designed the thing around gas discharge lights specially made for their spectra and had used fiber optics as light conduits to light his displays.
I give the owner 5-stars for creativity... this guy really knew his lighting. I didn't talk much about efficiency. I was so impressed on how well he used his light, along with the refraction of his jewelery, to make what I thought was the most eye-catching display in the mall.
I wonder how the astronomy people are going to take LED street lighting. I understand they were a big pusher of the HPS lighting because they could filter out the sodium spectra from their telescopes and minimize the effects of "light pollution". I guess Hubble renders the earth "light pollution" issue moot.
Some of the biggest present and future Earth-bound optical telescopes are fortunately sited in fairly remote locales, such as the top of Mauna Kea in HI and in the Chilean Atacama desert mountains.
http://en.wikipedia.org/wiki/W._M._Keck_Observatory
http://en.wikipedia.org/wiki/Magellan_Telescopes
http://en.wikipedia.org/wiki/Las_Campanas_Observatory
http://en.wikipedia.org/wiki/Giant_Magellan_Telescope
Hubble is the 1%. It's what catches the public's imagination (much like Bill Gates does), but the lion's share of astronomy is performed from terra firma, where we have vastly more glass on the sky (Hubble is a meager 2.4 meter aperture). LED spectra are certainly harder to deal with than line spectra, but I doubt we astronomers will hold much sway over the argument. Skies have only gotten brighter over time (watched this from Palomar, esp. in light of casinos popping up). So the astronomical community has not proven itself very effective at holding back the tide. Good shielding and judicious choices about where lighting is truly needed may be the best (compromise) tools.
Thanks, Tom, for an excellent article. I really hadn't understood the definition of a lumen before, and you also clarified some mysteries around CFL ratings.
I always appreciate the math, Tom. Finger food for the numerate.
It's curious there's no prior mention here of the revolution in daylighting that's happening with sun tubes. If you can poke a hole in the roof, these highly reflective tubes nominally 1 foot in diameter bring full spectrum sunlight into the deep caverns of modern cubicle spaces or dreary bathrooms, sometimes even one story below the roof. For the effort, they far exceed the merits of skylights, which are costly to install and typically put too much light and heat in one spot that moves around during the day.
Combining these 90% efficient light tubes with lighting controls, insulation and PV, we recently pulled a commercial retrofit down to ~10% of its previous electricity use.
No, the sun tubes don't work at night, but neither do the happy customers who appreciate the ultimate quality of full spectrum natural light.
Thanks for mentioning these. I had seen the totally inspiring youtube video on using soda bottles to make cheap light tubes in the Philippines. If any group is doing more with less to make the world a better place it has to be the folks behind Liter of Light.
But I did not know that tubular skylights were commercially available in the US form companies like Sun Dome.
Why would any new warehouse-type building not use these? Zero energy, zero maintenance, full spectrum light. Sun Dome even offers an add-on 10 W/1000 lumen bulb mounted inside the tube for work in the evenings.
There are so many ways in which we could use MUCH less energy than we do today!
Jon
> ... MUCH less energy...
Exactly. I see projections of increased energy use from all quarters. I would love to see curves showing increasing light, increasing mobility, increasing quality of life, and decreasing energy overall (e.g., like the refrigerators in California which have gotten lots of publicity).
We can deliver the same or more with 1/10th the energy; all we need is a metric to show the way!